
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN: 9781305079250
Author: Mark S. Cracolice, Ed Peters
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
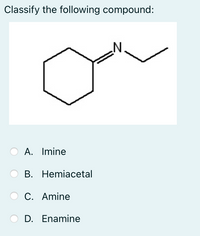
Transcribed Image Text:Classify the following compound:
A. Imine
В. Hemiacetal
C. Amine
D. Enamine
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Amide hydrolysis in basic conditions forms A. a carboxylic acid and an amine B. a carboxylate salt and an amine 3. an ester and an amine 4. a carboxylic acid and an amine saltarrow_forwardacetylsalicylic acid vs salicylic acid? What is the main chemical difference? Why can acetylsalicylic acid pass through the stomach without harm while salicylic acid can cause harm to the stomach????arrow_forwardPlease solve part Carrow_forward
- explain the solubility behavior of each representatives amine in water a. aniline b. diethylamine c. N,N-diethylanilinearrow_forwardWhat type of amine is this? 'N'arrow_forwardAmide formation involves which two functional groups? A.carboxylic acid and alcohol B.alcohol and ketone C.carboxylic acid and amine D.amine and alcoholarrow_forward
- pp.101edu.co New lab Question 18.a of 25 Describe the structure, bonding, and properties of this organic functional group. Choose the best classification for the compound shown. NH₂ A) amide B) primary amine C) secondary amine D) tertiary amine E) quaternary ammonium saltarrow_forwardWhat are the functional groups present in this antibacterial antibiotic? A. Amide, thioether, aldehyde, phenol, carboxylic acid B. Amide, thioether, ketone, amine, phenol, carboxylic acid C. Amide, thioether, ketone, phenol, carboxylic acid D. Thioether, ketone, amine, phenol, carboxylic acid A brief explanation would be highly appreciated + upvotearrow_forwardPredict the product of ester reacting with an excess of a primary amine under prolonged heating. A) unsubstituted amide. B) carboxylic acid and alcohol. C) monosubstituted amide. D) nitrile.arrow_forward
- The hydrolysis of an amide in acidic conditions forms A. a carboxylate salt and an alcohol B. a carboxylate salt and an amine C. an alcohol and an amine salt (an ammonium ion) D. a carboxylic acid and an amine salt (an ammonium ion)arrow_forwardDraw condensed and skeletal structures for each of the following amines a. 2-methyl-N-propyl-1-propanamine b. N-ethylethanamine c. 5-methyl-1-hexanamine d. methyldipropylaminee. e. N,N-dimethyl-3-pentanamine f. cyclohexylethylmethylaminearrow_forward1. Draw the structure for each compound and classify the amine as primary, secondary, or tertiary. a. dimethylamine b. diethylmethylamine c. 2-aminoethanolarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning