
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
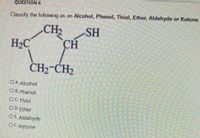
Transcribed Image Text:QUESTION 6
Classify the following as an Alcohol, Phenol, Thiol, Ether, Aldehyde or Ketone.
CH2
SH
H2C
CH
CH2-CH2
OA Alcohol
O B. Phenol
OC Thiol
O D.Ether
OE Aldehyde
OF. Ketone
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the skeletal ("line") structure of a secondary alcohol with 5 carbon atoms,1 oxygen atom, at least one ring, and no double or triple bonds.arrow_forwardWhat is the line structure and the functional groups present (if any)? a) cyclohexane b) cyclohexanol c) cyclohexanonearrow_forwardWhat is the IUPAC name of the ether that has the structure shown? This molecule has the condensed formula C H 3 C H 2 C H 2 C H 2 O C H (C H 3) 2. IUPAC name:arrow_forward
- a primary alcohol has one -OH group, a secondary hasarrow_forwardIn what functional groupings are the structures composed? ▸ B H&CH=CH₂ A) A ketone and an alkyne A) An alcohol and an alkyne B) A ketone and an ether B) An Aldehyde and a alkene A) A ketone and an alkene B) An alcohol and an alkene A) An Aldehyde and a ketone B) A ketone and an alkene CH3-CEC-CH₂OH ◆- Which type of bonding between carbon atoms in organic molecules is NOT typical? X₁ ◆ A functional component called the carbonyl group can be found in a wide range of substances. What's the carbonyl group's basic structure? CH A) -C- 8 C)--0)-C30 e) -4² ◆Which of the following statements about the two compounds is untrue? OH CH₂ - CH₂ - CH₂ Compound A A) Compound B has stronger intermolecular forces than compound A. B) Compound A and compound B are both organic compounds. C) Compound B has a higher boiling point than compound A. D)Compound A is Likely a gas at room temperature and compound B is likely a liquid at room temperature. E) Compound A and compound B are both hydrocarbons ◆ which…arrow_forwardWhat kind of functional group does the shown molecule have? A)Ether B)Ketone C)Aldehyde D) Carboxylic acid E) Alcholarrow_forward
- What would be the name of this organic molecule according to the IUPAC nomenclature?? H H H-C-H H-C-C-C--H H HI H H-C-Harrow_forwardWhich of the following is an ether functional group? O 8. H3C-C-O-CH3 OCH₂CH=CHCH₂CH3 O NH2 OCH₂CH₂-O-CH₂CH₂ aarrow_forward(a) Enter the IUPAC name for this alcohol: CH,CH,CHCH, ОН Classify it as a primary, secondary, or tertiary alcohol: (b) Enter the IUPAC name for this alcohol: CH,CHCH,CH,CHOH CH, ČH, Classify it as a primary, secondary, or tertiary alcohol:arrow_forward
- What is the description of a phenol and what is the description of a thiol? Which one contains either an alcohol or ether? and which one has a -SH functional group?arrow_forwardProvide the correct IUPAC name for the compound shown here. 2- 1- Question 26 of 30 sec- 5- 3- 4- 6- cyclo iso tert- tri di meth pent but eth prop hex ether acid yl Submit +arrow_forward11. Draw all of the alcohols and ethers of molecular formula C4H10O.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY