
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
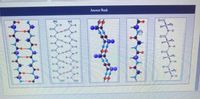
Transcribed Image Text:Answer Bank
NH
-R
R-C
N-H
H-N
H--R.
C-R
N-H
N-H
C-R
On
C-R
N-H
Transcribed Image Text:Resources
Classify cach peptide chain as part of a parallel ß sheet, part of an antiparallel B sheet, either type of B sheet, or not part
of a ß sheet.
Parallel ß sheet
Antiparallel ß sheet
Either
Neither
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 19. H,N-CH-Č-NH-CH, -COOH À BC D Ė Where is the peptide linkage in the dipeptide above? Oa. A Ob. B Ос. С C. C d. D Oe. Earrow_forwardWhich of the following amous indicates the peptide bond? CA) (B) (C) (D) (E) fut H₂N-CH-C-N-CH-C-OH CHPH H CH3arrow_forwardMatch the following in the picture abovearrow_forward
- ▼22. From each description, state whether the unknown solution contains an amino acid, a peptide, or a protein. a. An unknown solution gives a negative biuret test, but a positive xanthoproteic test. What may the unknown solution contain?arrow_forwardWhat is a cost and benefit to denaturing proteins (ie, a pro and a con)?arrow_forward1. Determine whether each of the following statements describes the primary, secondary, tertiary, or quaternary stucture of a protein. а. Peptide bonds join amino acids in a polypeptide chain. b. Two polypeptide chains are held together by hydrogen bonds. С. Hydrogen bonds form between adjacent segments of the backbone of the same protein to form a "folded fan" structure. 2. List the type of denaturing agent described by the following treatments: a. disrupting the disulfide bonds in hair to straighten it b. baking the proteins in dough to make breadarrow_forward
- Fibrous proteins peptide chains arranged in long strands, or fibers peptide chains highly folded into spherical shapes keratins collagens insoluble in water Globular proteins Answer Bank generally function in structure and support often function as enzymes or transport proteins water-soluble Both hemoglobinarrow_forwardDrag and label names to their appropriate location on the picture.arrow_forwardProteins 1 and 2 interact strongly. A significant part of the interaction is between the amino acid side chains shown below. Protein 1= arginine C6H14N402 . Protein 2 = glutamic acid C5H9NO4 A mutation occurs in protein 2 that changes the amino acid shown above to one of the amino acids shown below. What change should disrupt the interaction between proteins 1 and 2 the most? the least? Protein 2- aspartic acid C4H7NO4, protein 2= Lysine C6H14N20 Protein 2= serine C3H7NO3 and protein 2= leucine C6H13NO2 aspartic acid would be the most and leucine would be the least aspartic acid would be the most and serine would be the least lysine would be the most and serine would be the least lysine would be the most and aspartic acid would be the leastarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY