
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
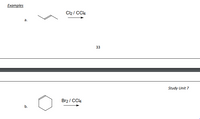
Transcribed Image Text:Examples
Cl2 / CCI4
a.
33
Study Unit 7
Br2 / CC4
b.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- k. F3C O i i CH3ONaarrow_forwardNonearrow_forwardP = P2O5 × 0.44 P2O5 = P × 2.29 K = K2O × 0.83 K2O = K × 1.21 1 tonne = 1,000 kg 1 ha = 10,000 m2 Using the conversions above calculate : 1.How much N and K are in a 25 kg bag of 12-22-18? 2. How much P2O5 and P are in a 20 kg bag of 0-46-0? 3. How much K2O and P2O5 are in a 35 kg bag of 12-22-18?arrow_forward
- E 8. h. CI CIarrow_forward5-10arrow_forwardInbox ( (531) C Conver I Balance b Answer Inbox CHEM8 101 Che X С Ч-С -с- bartleb ь Мy Qu Unknov Search E I make app.101edu.co/# Bryant's Gmail Cascadia Canvas Lo... T GSBA Scholarship L... HOMEGROWN TRA... Learn Touch Typing... C The Science of Well... Investor360° ® Login ClickUp Reading list >> Question 9 of 40 Submit Consider the intermolecular forces present in a pure sample of each of the following compounds: CH:CH:CH:NH2 and CH:CH2CO:H. Identify the intermolecular forces that these compounds have in common. A) Dipole-dipole forces and hydrogen bonding. B) Dipole-dipole forces only. C) Dispersion forces, dipole-dipole forces, and hydrogen bonding. D) Dispersion forces and dipole-dipole forces + 10:43 AM e Type here to search 64°F 8/26/2021 (8)arrow_forward
- 3. MF = CH10O CDCI3 240 QE-300 220 200 180 160 140 120 100 80 60 40 20 solvent 3. 2 12 11 10 9 6 4 1arrow_forwardAluminum-lithium (AI-Li) alloys have been developed by the aircraft industry to reduce the weight and improve the performance of its aircraft. A commercial aircraft skin material having a density of 2.65 g/cm? is desired. Compute the concentration of Li (in wt%) that is required. The densities of aluminum and lithium are 2.71 and 0.534 g/cm3, respectively.arrow_forwardalculate the approximate volume of the vinegar sample needed for the analyses (Part A.1). Brand of vinegar or unknown no. Colauita tof White Wine Trial 1 1122809 Trial 2 Trial 1 Trial 2 e Guia 112.280g (12.822g 112.796 115 565 lis.9 1. Mass of flask (g) l15.603 2. Mass of flask + vinegar (g) 2.496 3.323 2,743 2,749 3. Mass of vinegar (g) B. Analysis of Vinegar Sample 2mL 7.5 23mL 32.コ 1. Buret reading of NaOH, initial (mL) 5.25mL 13ML amL 2. Buret reading of NaOH, final (mL) 33,3 31.2 1. Volume of NaOH used (mL) 2! mL a7.45 me 20.3 32.2 o i135 • 004 1 Molar concentration of NaOH (mol/L) Oi135 • 00 3 1003 , 063 5. Moles of NaOH added (mol) 6. Moles of CH,COOH in vinegar (mol) 7. Mass of CH,COOH in vinegar (g) 8. Percent by mass of CH,COOH in vinegar (%) 9. Average percent by mass of CH,COOH in vinegar (%) Calculations for Trial 1 of the first vinegar sample on next page. Experiment 10 137arrow_forward
- 57.9 mL of 0.161 M Fe(NO3)3 and 24.7 mL of 0.557 M Ca(NO3)2 a) [ Fe3+ ] = 9.32 M; [ NO3- ] = 55.5 M; [ Ca2+ ] = 13.8 Mb) [ Fe3+ ] = 0.00932 M; [ NO3- ] = 0.0555 M; [ Ca2+ ] = 0.0138 Mc) [ Fe3+ ] = 0.161 M; [ NO3- ] = 3.59 M; [ Ca2+ ] = 0.557 Md) [ Fe3+ ] = 0.113 M; [ NO3- ] = 0.672 M; [ Ca2+ ] = 0.167 Me) [ Fe3+ ] = 0.0133 M; [ NO3- ] = 0.144 M; [ Ca2+ ] = 0.0460 Marrow_forward%24 4+ dl w/ b. Be sure to answer all parts. How many moles are contained in each number of grams of table sugar (C12H22011, molar mass 342.3 g/mol)? Enter your answer in scientific notation. a. 55.0 g b. 0.0250 g x 10 mol x 10 IIO %23 2 3. 5. 9- 8. 4arrow_forward1a. Determine the volume of 1.000 g of water (Densitywater = 0.9999 g/mL). 1b. Determine the volume of 1.000 g of ice (Density ce = 0.9168 g/cm³). (1 mL = 1 cm³) %3D %3D 1c. From the volumes above, what can we conclude about 1.000 g of water when it freezes?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY