
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:**Predict the monohalogenation products of these radical reactions, indicating major and minor products:**
---
This text serves as an introduction to a problem focused on predicting the outcomes of radical halogenation reactions. It asks for the identification of major and minor products for specific chemical reactions involving the addition of a halogen to organic molecules. Students are expected to apply their understanding of radical substitution mechanisms, taking into account factors like stability of reaction intermediates and the regioselectivity of the halogenation process.
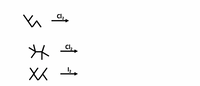
Transcribed Image Text:The image displays three chemical reaction equations involving hydrocarbons and halogens. Here is a detailed transcription and explanation suitable for an educational website:
1. **Reagent and Reaction:**
- Structure: The first structure is a hydrocarbon shown as a zig-zag line, representing a generic alkane.
- Reactant: Cl₂ (Chlorine gas)
- Reaction: The hydrocarbon reacts with chlorine gas.
2. **Reagent and Reaction:**
- Structure: The second line shows a branched hydrocarbon structure.
- Reactant: Cl₂ (Chlorine gas)
- Reaction: The branched alkane reacts with chlorine gas.
3. **Reagent and Reaction:**
- Structure: The third structure is a similar representation of a hydrocarbon chain.
- Reactant: I₂ (Iodine gas)
- Reaction: The alkane is reacted with iodine gas.
**Explanation:**
Each structure utilizes a skeletal formula to depict organic compounds, commonly used in organic chemistry to simplify the representation of molecules. The aim of these diagrams is to illustrate how different hydrocarbons undergo halogenation reactions. Halogenation typically involves the substitution of hydrogen atoms in an alkane with halogen atoms, resulting in the formation of different alkyl halides.
Expert Solution
arrow_forward
Step 1
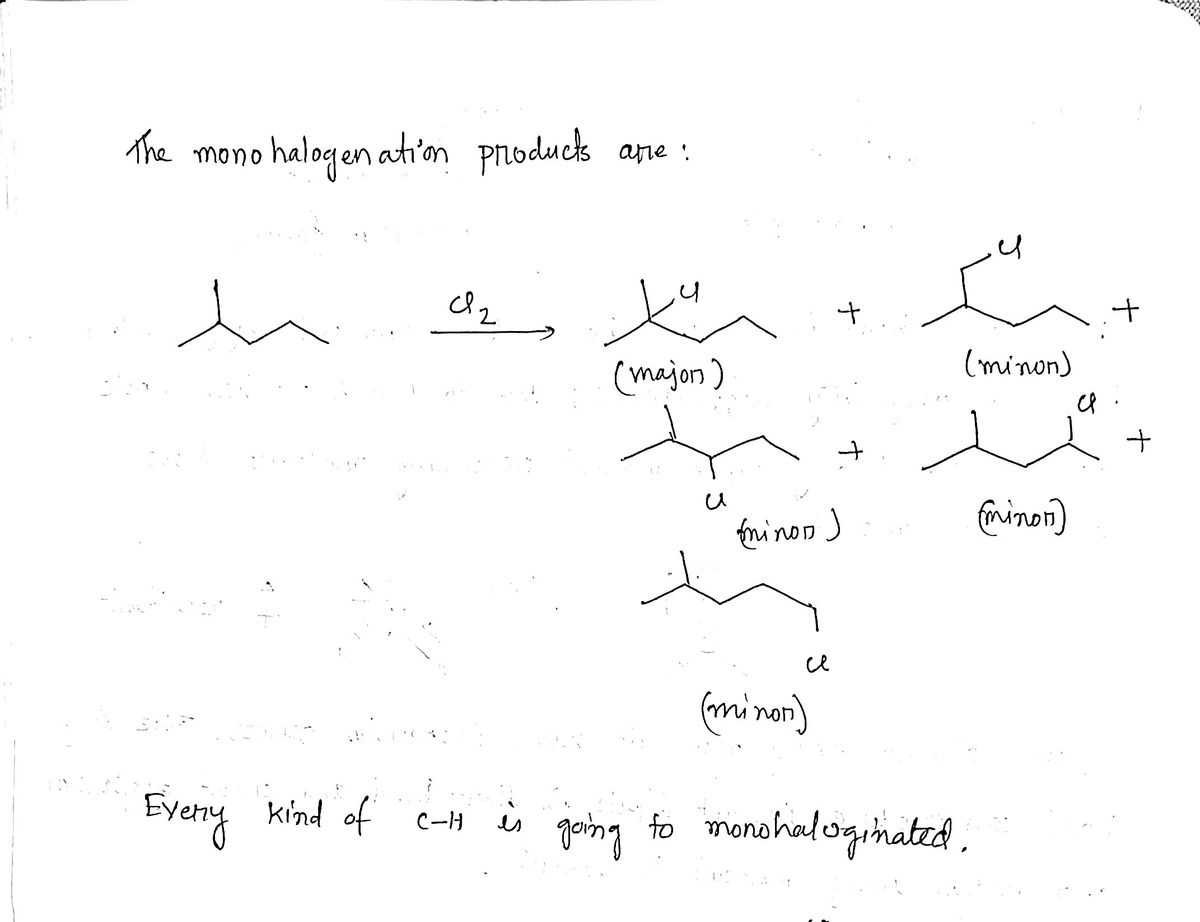
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Certain chemical reactions are endothermic while others are exothermic. In each of the following statements regarding a chemical reaction, state whether the reaction is endothermic or exothermic: (make sure to answer all 4 statements) a. ΔH has a positive value b. The energy of the reactants is greater than the energy of the products. c. Energy is released in the reaction. d. The bonds in the products are stronger than the bonds in the reactants.arrow_forwardExplain three examples that will significantly improve the accuracy of a calorimetry lab experiment.arrow_forward|The specific energy and density of gasoline is -47.3 MJ/kg and 716 kg/m³, respectively. What is its energy density? a. 0.066 mJ/m³ b. 33,867 MJ/m³ c. 0.066 m³/MJ d. 33,867 m³/MJarrow_forward
- ORT SHEET Heat of Neutralization EXPERIMENT elemsboomfchg lom 12 A. Heat Capacity of Calorimeter 1. Temp. of calorimeter and water before mixing 2. Temp. of warm water °C 22.0 39,0 30.3 3. Maximum temp. determined from your curve °C 4. Heat lost by warm water (temp decrease x °C 50.0 g x 4.184 J/K-g) = 02), 5. Heat gained by cooler water (temp. increase x 50.0 g x 4.184 J/K-g) = 30,3 22.0)x 13626J s0.0gmpi S0.0gy 6. Heat gained by the calorimeter [(4) – (5)] = 7. Heat capacity of calorimeter: heat gained by the calorimeter temperature increase J/K 3. Heat of Neutralization of HCl-NaOH 22.2 22.2. °C . Temp. of calorimeter and NaOH Temp. of HCI AT determined from your curve after adding HC1 °C to the NaOH Heat gained by solution (temperature increase x ON 100 g x 4.184 J/K-g) = 9977.8J %3D Heat gained by calorimeter (temperature increase x heat capacity of calorimeter) = J %3D Total joules released by reaction [(3) + (4)] = Tight O 2018 Pearson Education, Inc.arrow_forwardExothermic Endothermic Heat Heat Hotter than wOundings Heat Heat Heat Which statement is correct? O When an endothermic process takes place in the system, the entropy of the surroundings increases. O When an endothermic process takes place in the system, the entropy of the surroundings decreases. O When an endothermic process takes place in the system, the entropy of the surroundings does not change. O When an endothermic process takes place in the system, the entropy of the surroundings may increase or decrease.arrow_forward5. 'Н H H US LDA H₂O LDA H₂Oarrow_forward
- 6. An enthalpy change is a. the difference in the kinetic energy of the reactants and the products in a chemical change b. the difference in the potential energy of the reactants and the products in a chemical change c. the difference in enthalpies of the reactants and the products in a chemical change d. the sum of the potential and kinetic energies of the products e. the sum of the potential and kinetic energies of the reactants 7. Which statement concerning the Law of Conservation of Energy is not true? a. it applies to all chemical changes b. it involves all different forms of energy c. it applies to nuclear reactions d. it includes potential energy e. it involves heat content of substancesarrow_forward21. Which device to nutritionists use to determine the number of calories in samples of food? a foam cup filled with water a bomb calorimeter a spectrometer a constant pressure calorimeterarrow_forwardAll chemical reactions that are used as fuel must... O Have products higher in energy than reactants. O Have products lower in energy than réactant. O Have products with the same energy as the reactants.arrow_forward
- Enthalpy is the a. Heat of combustion at constant pressure b. Heat of formation at constant pressure c. Change in energy of a system at constant pressure d. Energy of a system at constant pressurearrow_forward2- Find the relationship between kcal and kJ and convert the energy values found in (1) to kJ.arrow_forward1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY