
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
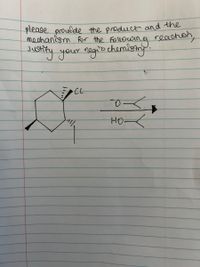
Transcribed Image Text:please proudde the product and the
mechanism For the Following reacton,
otity your negitechemistny.
HOK
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Linus Pauling (Recipient of two noble prizes one for chemistry and another for peace)Wrote a book, “Vitamin C and the common cold”, which propose that large doses of vitamin C can cure the common cold. One commercial over-the-counter product consist of 500.5 MG tablets that are 20.0% by mass vitamin C. How many of these tablets are needed for a 1.0 g dose of vitamin C? Each 500. Zero MG tablet cost two cents. If you can’t take a 1000. Zero MG tablet of pro vitamin C for $.13 a tablet, which would be more cost efficient ?arrow_forwardVinegar contains 5.00% acetic acid by mass and has a density of 1.01 g/mL. What mass(in grams) of acetic acid is present in 5.00 L of vinegar?arrow_forwardWhich of the following is a pure substance?? Air Sand Salt water Goldarrow_forward
- In the year 2013, an estimated amount of 36 billion metrictons (1 metric ton = 1000 kg) of carbon dioxide 1CO22 wasemitted worldwide due to fossil fuel combustion and cementproduction. Express this mass of CO2 in grams without exponentialnotation, using an appropriate metric prefixarrow_forward國 ① 14 14 1. 6C 7N+ 14 1N 1H 0. le 0. -learrow_forwardpls assist. thanksarrow_forward
- a white powder is cooled from the cruciblearrow_forwardThe definition of "Btu" is the energy needed to raise the temperature of 1 Ibm of water by 1 °F. The definition of "cal" is the energy needed to raise the temperature of 1 g of water by 1 °C. Find the unit conversion factor between "Btu" and "cal". Answer) 1 Btu =. cal Q = AH = m f'C,(T)dTarrow_forwardA chemist adds 800.0 mL of a 50.0 g/dL calcium bromide (CaBr,) solution to a flask. Calculate the mass in kilograms of calcium bromide the chemist has added to the flask. Be sure your answer has the correct number of significant digits. O kgarrow_forward
- Washington, D.C. has an average January temperature of 34.9 degrees Fahrenheit. What is this average if temp is expressed in Celsius? (Round to two decimal places.) [Hint: Conversion between Fahrenheit and Celsius is a linear transformation: Fahrenheit = Celsius*1.80 + 32, or Celsius = Fahrenheit*(1/1.80) - (32/1.80)]arrow_forwardOn the planet Xgnu, the most common units of length are the blim (for long distances) and the kryll (for shorter distances). Because the Xgnuese have 14 fingers, it is not perhaps surprising that 1400 kryll = 1 blim. Two cities on Xgnu are 21.2 blim apart. What is this distance in kryll?arrow_forwardPropylene glycol (C3H3O2) is a clear viscous liquid with a density of 1.04 g/mL. What is the volume of a 21.0-gram sample of propylene glycol?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY