
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
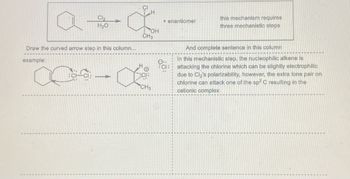
Transcribed Image Text:Cl₂
H₂O
Draw the curved arrow step in this column...
example:
1
H
H
OH
CH3
CH3
+ enantiomer
CI:
this mechanism requires.
three mechanistic steps
And complete sentence in this column
In this mechanistic step, the nucleophilic alkene is
attacking the chlorine which can be slightly electrophilic
due to Cl₂'s polarizability, however, the extra lone pair on
chlorine can attack one of the sp2 C resulting in the
cationic complex
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me with the organic chemistry problem below: Consider the reaction below: (Check the attached image) (it is between Furan and maleic anhydride, a DIels-Alder reaction) a) Will this reaction for an endo product (with a melting point of 80-81 degrees) or the exo product (with a melitng point of 114 degrees)? b) Carefully explain why the product must have been formed the way it did (exo or endo). c) Provide a mechanism for this reaction.arrow_forward1.arrow_forwardReview Topics) [References] Draw the products of the reaction shown. Electron flow is indicated with curved arrows. H.C. H :Br: CH₂ :OCH3 Include all valence lone pairs in your answer. ■Include counter-ions, e.g., Nat. I, in your submission, but draw them in their own separate s Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the right corner. Separate multiple products using the + sign from the drop-down menu. 85arrow_forward
- Q;Provide missing curved arrows and draw the product of the radical addition mechanistic step. Add lone pairs and radicals to all appropriate species. Provide missing curved arrows. Make sure to use the correct curved arrows.arrow_forwardNoting the curved arrows, draw all the product(s), organic and inorganic, of the following reaction. H. H H3C-C-CH3 0-CH3 • You do not have to consider stereochemistry. ● Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate structures with + signs from the drop-down menu.arrow_forwardOrganic Chemistry Problem. Please help with reaction question. Thank you.arrow_forward
- Don't use hand raiting pleasearrow_forwardPlease don't provide handwritten solution ....arrow_forwardPart A Propose a mechanism for the free-radical chlorination of ethane. hv CH; -CH, + Cl, CH; -CH,CI + HCI Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. Include all free radicals by right-clicking on an atom on the canvas and then using the Atom properties to select the monovalent radical. H CI Br Marvin JS by ChemAxon Submit Request Answer 1 0 +f .arrow_forward
- Follow the flow of electrons indicated by the curved arrows in the following polar reaction. Draw all products that result, including any inorganic ions. H₂0 H₂C ** CH3 • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Include all valence lone pairs in your answer. ChemDoodle Previouarrow_forwardThis organic molecule is dissolved in an acidic aqueous solution: i A short time later sensitive infrared spectroscopy reveals the presence of a new C. with at least one C - OH bond. - OH stretch absorption. That is, there must now be a new molecule present In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule. : Click and drag to start drawing a structure. Add/Remove step Click and drag to start drawing a structure.arrow_forwardDraw both the organic and inorganic intermediate species. Include nonbonding electrons and charges, where applicable. Include hydrogen atoms.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY