
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Draw the product(s) of the following reactions. Specify stereo and regio-selectivity where appropriate. If more than one product forms indicate major and minor.
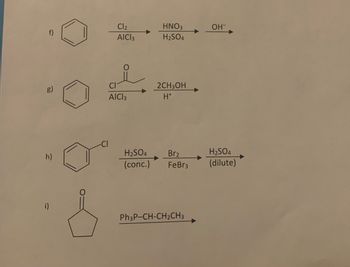
Transcribed Image Text:g)
h)
i)
Cl₂
AICI 3
AICI 3
HNO3
H₂SO4
2CH3OH
H*
H₂SO4 Br₂
(conc.) FeBr3
Ph3P-CH-CH₂CH3
OH-
H₂SO4
(dilute)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Involves a carbocation intermediatearrow_forward[References] Alkyl halides undergo nucleophilic substitution and elimination reactions. When the kinetics of the reaction are measured, if the rate of the reaction is found to be dependent only upon the concentration of the alkyl halide the reaction is first order. The substitution reaction is thus termed SN1, and the elimination reaction is termed E1. These reactions are unimolecular and occur in two steps. The first step is rate-limiting and involves the loss of the leaving group to form a carbocation. In the second, fast, step the nucleophile adds to the carbocation in the SN1 reaction or elimination occurs to give an alkene in the E1 reaction. Because the carbocation is planar, the nucleophile can add to either face and therefore racemization is usually observed although solvent effects can influence this somewhat. E1 elimination follows Zaitsev's rule and typically yields the most substituted alkene as the major product. Conditions which favor the SN1/E1 pathway include the use of…arrow_forwardQ5. Give the major organic product(s) or missing reagent(s) of each of the Sollowing reactions or sequences of reactions. Show all relevant stereochemistry (22 Marks). COCH NO₂ (CH/00-0 SWOHL, 5.0 H-SO₂ CH₂C AICI, benzene AKCarrow_forward
- Give the structure of the product and/or intermediates of the following reactions. Indicate, where appropriate, both regiochemistry and stereochemistry.arrow_forwardCould you please also explain and draw out the electron movement throughout the reaction.arrow_forward[Reference Draw a structural formula for the major organic product of the reaction shown below. C(CH3)3 + CH₂ (2) a H3C 2 • You do not have to consider stereochemistry. CuLi ether H₂O +arrow_forward
- Complete the following reaction scheme. Give all product(s) and indicate major or minor and any relevant stereochemistry (G) H2 CH2 H2 -CH2 Pd/C CH H2C (k) OH ....OH OsO, NMO OH (1) BI2arrow_forwardPlease don't provide handwritten solution...arrow_forwardb) Ô s) Write in ALL reasonable products of these reactions. La -CI benzene H3C H₂C AICI, CH3 NH₂ Br₂ FeBr CH3 Br₂ FeBr3 NaBH₁ HNO,arrow_forward
- Predict the major organic product for the following reaction. You do not need to account for stereochemistry, but be sure your answer accounts for regiochemistry where appropriate. Assume cis conformation of the diene. HC=CH Product?arrow_forwardPlease don't provide handwritten solution ....arrow_forward4.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY