
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
fill the blanks
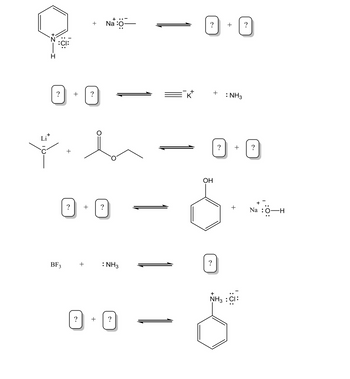
Transcribed Image Text:Li
BF 3
+
+ ?
+
+
+
+
Na :O
: NH3
?
?
+
OH
+ : NH3
0+ 0
+
+
NH3 CI
:C:
Na :0—H
-H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Uranium has three isotopes : uranium-233, uranium-235, and uranium-238. Compare the number of neutrons in each isotope. Determine which isotope is the most common and explain how you know .arrow_forwardWhich pair of elements have the most similar chemical properties? Question 1 options: Na & K Si & Ne N & Farrow_forwardQ 37arrow_forward
- Fill in the blanks below with a maximum of two words in each space to complete the following definition or principle: Note for filling in blanks: • If one word completes that part of the statement, do not include alternate or additional words. Two words should only be used when two words are needed to complete that part of the statement. Alternate and additional words will be marked as incorrect. • Do not use abbreviations or short-forms of words. • Spelling will be graded in the submitted words. Le Châtelier's Principle defines how a system at will react to an imposed change until it is able to A equilibrium.arrow_forwardwhere did you get 10^-14?arrow_forwardWhich of the following is a chemical property? O Changing shape Melting point Oxidizing Explosiveness Sublimationarrow_forward
- How many cm3 are there in 2.11yd3?arrow_forwards 15 Date x Cross Check Circle Erase Tools (9) Replace text Comments O Search Fit Width Help Feedback To enter data on this form, use the fillable fields or annotation tools. 1. Osmium metal, the densest element, has a density of 22.6 g/mL, while hydrogen, the least dense element, has a density of 8.99 x 105 g/mL. Calculate the volume occupied by 1.00 g of each element. How many times more dense is osmium than hydrogen? Show all of your work. Sign out UFarrow_forwardPed 1 2 3 5 6 新 1 H 11 12 Na Mg 4 1223 Chapter 2: Chemistry Comes Alive 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 4 Be 55 2&4 44 AS 263992943 45 47 48 49 50 5 A 40 Ru Cd Ag 292 73 735 W 25 677 78 20 Pt Au Re 88 474 25 26 27 2 Mn 57 La 41 Nb Mo . 951 Hg 89 Mc 108 109 110 111 112 113 114 115 128 22:104 105 106 107 116 11 Ts Lv Og Ra 58 61 62 63 59 500 Ce Pr Nd Pm Sm Eu 91 82787 27 15 15 16 Totu 90 99 96 93 Md Es No -9222 ²23 24 25 26 27 29 23 100 101 102 103 Th Np 64 Match each statement with a response chosen from the key. Key: Solution Colloid Suspension 1. blood 2. water 3. milk or jell-o 4. sand in water 25 He 10 Ne 18 26 36 Kr 67 68 69 Tm 20 86 Rn 5. solute particles do not settle or scatter light 6. solute particles are large, settle out, and may scatter light 7. solute particles are larger than in a solution, scatter light, and do not settle outarrow_forward
- Given the symbolic notation below: 15 X3 7 Which of the following choices correctly represents the numbers on the symbolic notation above? O A. 7 is the atomic number, the element is nitrogen, the mass number is 15, the amount of neutrons is 8, and the atom lost 3 electrons. O B. 15 is the atomic number, the element is potassium, the mass number is 7, the amount of neutrons is 22, and the atom gains 3 electrons. O C. 7 is the atomic number, the element is nitrogen, the mass number is 15, the amount of neutrons is 8, and the atom gains 3 electrons. O D. 15 is the atomic number, the element is potassium, the mass number is 7, the amount of neutrons is 22, and the atom lost 3 electrons. novo %23 4 е r f C V barrow_forwardWrite a complete chemical formula, including physical state symbol, for each element listed below in its standard state. The first row of the table has been completed for you. element iron carbon hydrogen krypton I Don't Know formula Fe(s) 0 Submit X 00 $ S n Stv © 2023 McGraw Hill LLC. All Rights R 9 A Ⓒ Jarrow_forwardN Netflix How do the properties of compounds compare to the properties of the elements from which they are derived? The properties are generally different. The properties are the same. The properties are different only for metals. The properties differ insignificantly.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY