
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
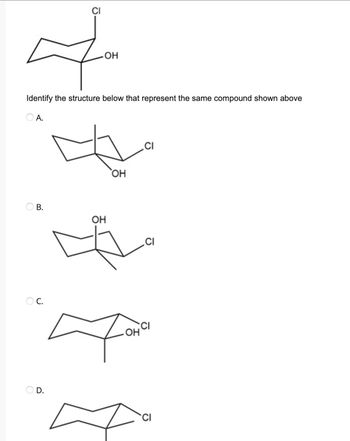
Transcribed Image Text:Ф
-OH
Identify the structure below that represent the same compound shown above
O A.
B.
OC.
OH
OH
.OH
CI
.CI
CI
Expert Solution
arrow_forward
Step 1
In this given molecule Ring flipping takes place.
It is a interconversion of cyclic conformersi.e, chair form (cyclohexane)
After ring flipping substituents attach to carbon 1 are moves one place forward and take a position on carbon 2. & So on
In simple words substituents attached carbon is shift & take a position of next carbon.
After ring flipping , substituent which is present in axial position is changed into equitorial position & vice versa. But the direction or stereo is retained.it means before flipping OH is in equitorial position & it's direction is upward (wedge), But after flipping , it convert into axial position with upward direction (wedge).
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4. Provide the functional groups, excluding alkanes, present in each molecule. Identify all 4. Provide the functional groups, excluding alkanes, present in each molecule. Idetny an 4° carbons and all 1° hydrogens.arrow_forwardL. FORMULAS Identify and determine the types of formulas used in the given structures as follows: A. Molecular Formula B. Expanded Structural formula C. Condensed structural Formula D. Line bond CH3 CH;CHCHCH,CH; ČH,CH, ČI 1. ннн нн CH,CH3 H-C-C-c-C-C-H CH H2C CH2 H H H H H CH2-CH2 3. 4. CH,CH,CH,CHBICH,CH3 CH,CH=CH2 C3H8 5. 6. 2.arrow_forwardHow many of the following molecules will rotate plane polarized light? H. CH, CH3 Me H. CH, CH,CH3 H;C. CH2CH3 H;C. H. ČH, Br cı' H H;CH,C 2arrow_forward
- 15. a. Draw two constitutional isomers with the formula CaHsBrCl ( Isomer 1 Isomer 2 b. Describe ALL the intermolecular forces isomer 1 can participate in 16. For the molecule below, OH (a) Circle all functional groups present. (b) Label two of the functional groups next to where you circled them 3arrow_forward2 O L 40% A moodle.lsua.edu Alanie Fontenot my.LSUA HEM 1001/LEC/O01X-2021/SPRING/SEM - ro Chemistry for Non-Science Major I e / My courses / 2021 | 2021/SPRING / CHEM 1001/LEC/001X-2021/SPRING/SEM / Chapter 4 Functional Groups estion 3 What is the functional group found in the following molecule? t yet H H. swered pints out of H -C-C C - Flag uestion 0-C- C-H Select one: a. amide O b. carboxylic acid O c. ester O d. ether O e. ketone HIU- I- ООО О Оarrow_forwardConvert each skeletal structure to Kekulé structure. OH HO a. С. `N' menthol (isolated from peppermint oil) ethambutol (drug used to treat tuberculosis) OH b. d. myrcene (isolated from bayberry) HO estradiol (a female sex hormone) IZarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY