
Pushing Electrons
4th Edition
ISBN: 9781133951889
Author: Weeks, Daniel P.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Label each C–C double bond as E or Z.
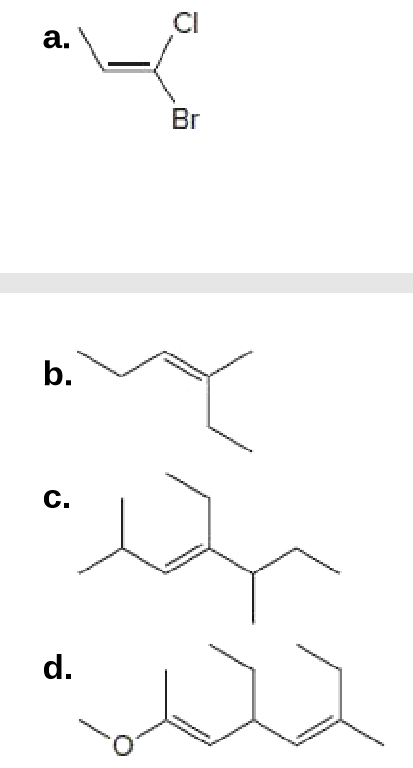
Transcribed Image Text:CI
a.
Br
b.
C.
d.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- "Gatorade contains sodium and potassium electrolytes that can help to replenish ions that are lost as a result of dehydration. a. b.c. d. e. f. g. h. I. j. k."arrow_forwardABCDE A. B. C. D. E. A A B (C) C D B E E 1. Br2, CC14 1. H₂, Lindlar's cat.; 1. Br2, CC14 1. Na, NH3(0); 1. Br2, CC14 Br CH₂CH3 Br H 2. H₂, Lindlar's cat. 2. Br2 2. Na, NH3(1) 2. Br2 2. H₂, Pt + enantiomerarrow_forward8. Fill in the following boxes. . HO. heat HO. SOC2 NaN3 1. he t 2. H2C 2 Mel +arrow_forward
- i. C₁7H36 plus oxygen gas j. magnesium metal plus silver nitratearrow_forward5.) Correctly match the terms listed on the left with the substances listed on the right. More than one term may be used for each substance. A. element B. compound C. heterogeneous Mg Fe mixed with sand saltwater D. mixture water E. homogeneous F. pure substance G. Solution M9SO. 41 8 3. 5 COarrow_forwardThe molecular orbital (MO) diagram of OF is given below. Answer Questions 15 – 18. 2p, 2p, 2p, 2p. 2p, 2p. 25 25 OF Farrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you


 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning



Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning