
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
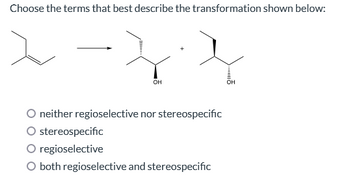
Transcribed Image Text:Choose the terms that best describe the transformation shown below:
OH
neither regioselective nor stereospecific
stereospecific
regioselective
O both regioselective and stereospecific
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Choose the best description for the selectivity/specificity of the transformation shown below: O regioselective stereospecific OH Save for Later HO neither stereospecific nor regioselective both stereospecific and regioselective OHarrow_forwardFor each organic compound in the table below, enter the locant of the highlighted side chain. compound CH 3 | CH₂ - CH - CH₂ - C - CH₂ 1 1 CH3 - CH₂ I CH3 CH3 CH₂ CH3 t CH₂-C- CH₂ - CH - CH₂ CH3 CH3 1 CH3 CH₂-C- | CH3 CH3 locant of highlighted side chain 0 3arrow_forward11. Choose the Fischer projection that is the structure shown. Н ОН H3C H3C- F H A -ОН 애 -OH F НО- H3C- -Н ОН 00 в -CH3 H3C НО НО CH3 F OH CH3 -F -Н CH3 C I CH3 -OH -CH3 ОН D H3C- ОН -CH3 -Н ОН Earrow_forward
- Knowing that the stereocenter is S, why does the top circle beat the bottom circle in priority since the top circle is only conected to an OHH and the bottom ciurcle is atttached to a N and a C?arrow_forwardWhich of the following are meso compounds? CH3 CH3 CH3 CH3 CH H- CI H CI -H CI- H CI- -CI H- -H H- H H- -H H -H H- -H H- -CI -H H H -CI H- -H CH3 CH3 CH3 CH3 CH3 D E A Barrow_forwardAssign 1,2 and 3 configuration S or Rarrow_forward
- Provide the least stable conformation of the following compound. Cis/trans stereochemistry mattersarrow_forwardWhich set of Newman projections represents correct staggered and the eclipsed least stable conformation for the following compound: Н H3C H тос Н- CH3 H3C O A 000 A ОВ CH3 CH3 Н H Н Å Å Å Å H H Н CH3 H3C CH3 CH3 CH3 CH3 Ос OD H3C H3C- 00 В CH3 H3C Н Н Н CH3 H3C. с Н ___H то H- CH3 CH3 Н- D H3C CH3 Н Н CH3arrow_forwardWhich Newman projection corresponds to the conformation of this compound that can undergo E2 elimination? Et CI E2 ? H tBu tBu tBu H H Me Et. H Me H CI tBu CI H CI H CI H Me Et Me Et A B Darrow_forward
- Select the correct Newman projection looking down the C2-C3 bond of the below structure. H H₂C O A OB O C D CH₂CH3 CI CH3 A Br CI Br CH₂CH3 H CH₂ H₂C Br CH3 CH₂CH3 H * H₂C Br CH3 C H₂C H CH₂ Br CI CH₂CH3arrow_forwardWhat is the enantiomer of compound Y? NH2 Compound Y NH2 Br NH, ÑH2 Br ÑH2 Br II II IV NH2 Br Br NH, V VI O lonly O V only O IV only III and V O Il only O VI only Il and II O Il only O None of the abovearrow_forwardAssign every stereo center in callyspongiolide as either R or S using CIP notationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY