
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
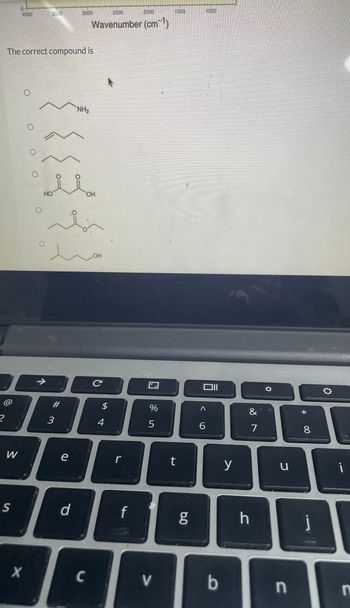
Transcribed Image Text:2
W
0
4000
S
The correct compound is
X
O
O
3500
O
HO
3
e
NH₂
han
d
2500
2000
Wavenumber (cm-¹)
OH
C
LOH
с
$
4
r
f
%
5
V
1500
t
g
1000
A
6
b
y
h
7
u
n
*
8
i
n
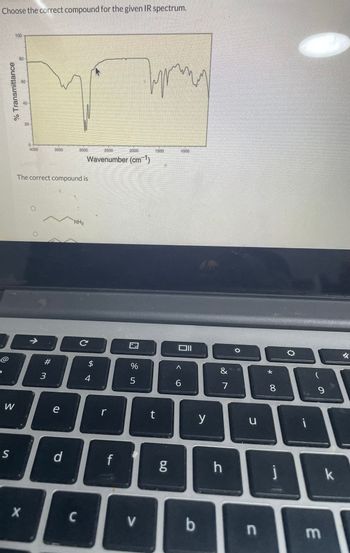
Transcribed Image Text:Choose the correct compound for the given IR spectrum.
% Transmittance
@
W
S
100
80
R
X
4000
→ ·
#
3500
The correct compound is
3
e
d
3000
NH₂
C
Wavenumber (cm¹)
с
LA
4
r
f
%
5
V
1500
t
g
1000
^
6
b
y
&
7
h
O
u
n
* 00
8
j
i
9
m
k
✓
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain in detail:arrow_forwardLabel the major peaks, especially those those related to fumaric acidarrow_forwardCalculate the energy separation in wavenumbers (as m-1) between the levels n = 2 and n = 3 of an electron in a box of length 12 angstroms. O 1.053x10-6 m-1 O 3.159 x10-8 m-1 O 0.949x106 m-1 O 1.053x10-9 m-1 O 3.159 x10-6 m-1arrow_forward
- The Carbon monoxide molecule, CO, has an energy difference of 4.77 x 10 -4 eV. The wavelength of the transition from l=0 to l=1 rotation levels is 2.6 mm. Determine the bond length r0 of the CO molecule in nanometers with three decimal places.arrow_forward2) a) Convert the absorption value of 0.173 into % transmittance b) Convert the transmittance value of 22.7% into absorbance c) Calculate the frequency in Hz, the energy in joules and the energy in electron volts of an Xray photon with a wavelength of 5.47 Angstrom.arrow_forwardConvert the following Transmittance to absorbance: T=0.305arrow_forward
- What will the frequency of a modulated signal from a Michelson interferometer with a mirror velocity of 1.29 cm/s for radiation with a wavelength of 429 nm?arrow_forward1/ Please explain the answer in detail The J = 0 to J = 1 transition for carbon monoxide (12C160) occurs at 1.153 x 105 MHz. Calculate the value of the bond length in carbon monoxide.arrow_forwardDopamine absorbs light at a λmax of 265 nm. A sample of dopamine (molar mass 153.18 g/mol) was prepared by dissolving 1.02 mg of dopamine in enough water to make a 500.00 mL sample. The UV absorbance at 280 nm was found to be 0.450 in a 1.500 cm cuvette. What is the molar absorptivity of dopamine in L mol-1 cm-1?arrow_forward
- • O-H in alcohols and phenols, N-H 3200-3600 • O-H in acids 2400-3600 • C-H vinyl or aromatic 3000-3100 • C-H alkyl 2800-3000 • C=C and C=N 2100-2260 • C=O 1670-1750 • Alkene C=C 1620-1680 • Aromatic ring C=C 1600, 1500, 1450 (2 or 3 bands) Which compound in this list corresponds with the IR spectrum shown? OH HO. ČH3 CH3 Aarrow_forward4. Is the C=O stretching frequency the same for acetone and deuterated acetone? Explain your answer. Identify C O overtone in acetone spectrum which corresponds to the transitionn from ground level n= 0, to the second excited levarrow_forwardSagararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY