
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Choose the correct answer(s) for the equation for the reaction between aqueous solutions of hydroiodic acid and potassium hydroxide .
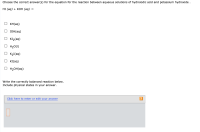
Transcribed Image Text:Choose the correct answer(s) for the equation for the reaction between aqueous solutions of hydroiodic acid and potassium hydroxide .
HI (aд) + КОН (ад) —
KH(aq)
IOH(aq)
KI2(aq)
O H20(1)
K2I(aq)
KI(aq)
O H2OH(aq)
Write the correctly balanced reaction below.
Include physical states in your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Describe the characteristics of a neutralization reaction, how it is identified, and types of compounds in the reactants and products. Give an example in your Response.arrow_forwardConsider two aqueous solutions: one of ammonium carbonate and one of barium nitrate. List the ions present in each. Is a precipitate formed when the two solutions are mixed? If so, write the molecular, ionic and net ionic equation. Identify the spectator ionsarrow_forwardDefine the terms acid and basearrow_forward
- Enter your answer in the box provided. A 2250 mL sample of 2.50 M HCl solution is treated with 4.14 g of magnesium. Calculate the concentration of the acid solution after all the metal has reacted. Assume that the volume remains unchanged. M HCI encesarrow_forwardA 20.0 mL solution of NaOH is neutralized with 31.1 mL of 0.200 M HBr. What is the concentration of the original NaOH solution?arrow_forwardHow many grams of solid calcium hydroxide are needed to exactly neutralize 17.4 mL of a 1.30 M nitric acid solution ? Assume that the volume remains constantarrow_forward
- give an example of neutralisation reaction.arrow_forwardComplete the equation for the neutralization reaction between solutions of hydrochloric acid and potassium hydroxidearrow_forwardCarbonated beverages are created when carbon dioxide gas is mixed with sugary liquids. Is the carbon dioxide gas the solute or the solvent of this solution? solute solventarrow_forward
- Consider two aqueous solutions: one of lithium hydroxide and one of calcium chloride. List the ions present in each. Is a precipitate formed when the two solutions are mixed? If so, write the molecular, ionic and net ionic equation. Identify the spectator ions.arrow_forwardIs the acid-base neutralization reaction between sodium hydroxide and hydrochloric acid an exothermic or endothermic process?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY