
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
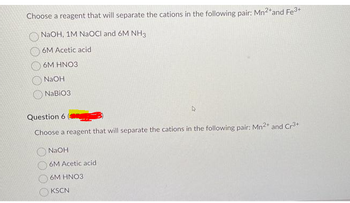
Transcribed Image Text:Choose a reagent that will separate the cations in the following pair: Mn²+ and Fe³+
NaOH, 1M NaOCI and 6M NH3
6M Acetic acid
6M HNO3
NaOH
NaBiO3
Question 6
Choose a reagent that will separate the cations in the following pair: Mn²+ and Cr³+
NaOH
6M Acetic acid
6M HNO3
KSCN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 16.71 What is the pH of a solution that is 0.600 M HCHO2 (formic acid) and 0.400 M NaCHO₂? 16.72 What is the pH of a solution that is 0.20 M KOCNarrow_forwardWhat is the HClHCl concentration if 49.2 mLmL of 0.100 MM NaOHNaOH is required to titrate a 20.0 mLmL sample of the acid?arrow_forwardWhich of the following would be a salt used in a buffer solution? OCH3COONa O NaCI O H2CO3 о кОнarrow_forward
- NH3(aq)+H2O⇄NH4+(aq) + OH-(aq) What happens to this equlibria in regards to Le Chateliers principle when HCL was added? This caused the indicator and solution to turn from pink to clear.arrow_forwardDetermin the pOH for a 1650 mL solution of 3.00 g of Mg(OH)2arrow_forwardCalculate the pH of a titration between 15.0 mL of 0.10 M HCl and 8.0 mL of 0.20 M NaOH at 25°C.arrow_forward
- What is the concentration of hypochorite ion if 20.00 mL of bleach requires 32 mL of 0.500 M HCl to reach the equivalence point?arrow_forwardIn an acid base titration between Carbonic acid and Sodium hydroxide, which of the following salts is not formed? Na2CO3 NaHCO3 NaH2CO3arrow_forwardWhat is the pH of an aqueous solution containing 75.0 mL of a 0.15M HNO2 solution and 45.0 mL of a .25M KNO2? What is the pH of this solution after 10.0 mL of a 0.20M solution of KOH is added? How would you categorize this problem (i.e. strong acid or weak acid titrated by strong base or weak base, buffer)?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY