
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
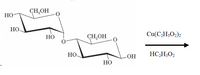
. If a reaction will occur, give the product(s) otherwise write no reaction. Also indicate if the reaction is fast or slow. Explain your answer.
Transcribed Image Text:CH,OH
HO-
НО.
Но
CH,OH
Cu(C,H3O2)2
Но.
HC,H;O2
Но-
НО
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- energy (J) A B Iarrow_forwardAt a particular temperature, if a reaction is a reactant-favored process what is true about this reaction? a. reactants predominate over products. b. reactants rapidly react with one another to form products. c. products predominate over reactants. d. products rapidly react with one another to form reactants. e. none of these choices.arrow_forwardHow I do thisarrow_forward
- Pick a reversible reaction, and explain the processes that occur in both directions of the reaction. Use the paperclip button below to attach files.arrow_forwardlist several attributes that are used to compare the “greenness” of a solvent and explain why catalyst are useful in making reaction greenerarrow_forwardAn equilibrium-controlled reaction will yield: Select one: ⒸA. the product that forms the fastest. ⒸB. the product whose formation involves the smallest energy of activation. OC. the product that can be formed in the fewest steps. OD. the most stable product.arrow_forward
- Which state of matter typically reacts the slowest in combination reactions? a. solid b. liquid c. gas d. aqueousarrow_forwardplease list the cauculusion step one step specificly, thank you so much!arrow_forwardHeroin and morphine (shown below) are odorless solids. Drug-sniffing dogs can sometimes locate heroin that has been exposed to the elements because of a strong odor that is present in small quantities. What compound causes the odor and suggest what reaction is responsible for the compound's formation. H3C. но reaction? odor compound? 'N. CHз CHз H3C но herion morphinearrow_forward
- How do you know what power to raise the product and reactant to in the Nerst equation?arrow_forwardConsider this potential energy diagram Which diagram represents the effect of a catalyst on the same reaction?arrow_forwardUsing the concept of collisions and molecular motion, explain why mixing a reaction mixture can speed up the rate of reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY