
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please answer #10 on attachment.
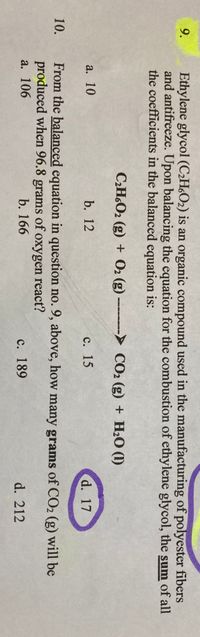
Transcribed Image Text:Ethylene glycol (C2HO2) is an organic compound used in the manufacturing of polyester fibers
and antifreeze. Upon balancing the equation for the combustion of ethylene glycol, the sum of all
the coefficients in the balanced equation is:
9.
CH,O2 (g) + O2 (g) CO2 (g) + H2O (1)
a. 10
b. 12
с. 15
d. 17
From the balanced equation in question no. 9, above, how many grams of CO2 (g) will be
produced when 96.8 grams of oxygen react?
a. 106
10.
b. 166
с. 189
d. 212
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- MCPBAarrow_forward33. Aldosterone is an example of a hormone. Oa. glucocorticoid ob. mineralcorticoid C. sex Od. prostaglandinarrow_forwardWhich of the following statements concerning fats and oils are incorrect? 1. They are also called as triacylglycerides. II. They are acidic in nature. III. They serve as storage form of fatty acids in animals and plants, respectively. IV. They are liquid and solid at room temperature, respectively. Select the correct response:arrow_forward
- What products would form from the reaction shown below? H. HO. TWO PRODUCTS HO, HO, HO. OH но. OH OHarrow_forwardWhich of the following factors primarily dictates the distribution of terrestrial biomes on Earth? The history of human development and civilization ㅎㅎㅎ The population density of animals Climate variables like temperature and precipitation The prevalence of certain rock and soil typesarrow_forwardIdentify the definition of each term. Term 00 Definition are triglycerides that are liquid at room temperature. are fats, oils, and other fat-soluble molecules. contain a carboxylic acid group and a hydrocarbon chain. are molecules that have three ester functional groups. are triglycerides that are solid at room temperature. Answer Bank triglycerides lipids oils fats fatty acidsarrow_forward
- 13. Some fungi live as single-celled organisms. Group of answer choices True False 14. For a fungus, a fruiting body such as a mushroom serves which function? Group of answer choices Allowing asexual reproduction by fragmentation Spreading spores produced during sexual reproduction Causing disease by consuming tissues in an animal host Storing nutrients as a permanent organ in the fungus Question 15 What sort of diseases can be treated with antibiotics? Group of answer choices Cancers Diseases caused by viruses Diseases caused by fungi Diseases caused by bacteria Question 16 Which of the following is an example of a bacterium causing harm to another organism? Group of answer choices Tuberculosis in humans caused by Mycobacterium tuberculosis Protozoa in the genus Plasmodium causing malaria in humans Fungi in the genus Penicillium producing penicillin that kills nearby bacteria The ghost plant Monotropa uniflora stealing nutrients from fungi NOV 14 MacBe esc 80 O00 000 F1 F2arrow_forwardWhich of the following is inorganic? vitamins water lipids carbohydratesarrow_forwardThe condensed structural formula for a wax is shown below. Type the condensed structural formulas for the two reactants that react to form this wax. || CH3-(CH2)14=c-0-(CH,)29–CH3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY