
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
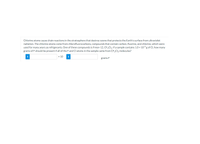
Transcribed Image Text:Chlorine atoms cause chain reactions in the stratosphere that destroy ozone that protects the Earth's surface from ultraviolet
radiation. The chlorine atoms come from chlorofluorocarbons, compounds that contain carbon, fluorine, and chlorine, which were
used for many years as refrigerants. One of these compounds is Freon-12, CF2CI2. If a sample contains 1.0 x 10-9 g of CI, how many
grams of F should be present if all of the Fand Cl atoms in the sample came from CF2Cl2 molecules?
x 10
i
grams F
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Aqueous hydrobromic acid HBr reacts with solid sodium hydroxide NaOH to produce aqueous sodium bromide NaBr and liquid water H2O . If 0.321g of water is produced from the reaction of 3.2g of hydrobromic acid and 2.1g of sodium hydroxide, calculate the percent yield of water.Be sure your answer has the correct number of significant digits in it.arrow_forwardDraw the major elimination product formed in the reaction. Select Draw Rings More Erase C H CH;O-K* DMSOarrow_forwardWhat is the easiest process to get to the result?arrow_forward
- Visited Hydrocarbons, compounds containing only carbon and hydrogen, are important in fuels. The heat of combustion of cyclopropane, C3H6, is 499.8 kcal/mol. Write a balanced equation for the complete combustion of cyclopropane. + How much energy is released during the complete combustion of 444 grams of cyclopropane ? kcalarrow_forwardEthanol, C,H,O, is most often blended with gasoline - usually as a 10 percent mix - to create a fuel called gasohol. Ethanol is a renewable resource and ethanol-blended fuels, like gasohol, appear to burn more efficiently in combustion engines. The heat of combustion of ethanol is 326.7 kcal/mol. The heat of combustion of 2-methylheptane, C3H18, is 1.306×10³ kcal/mol. How much energy is released during the complete combustion of 482 grams of 2-methylheptane ? kcal Assuming the same efficiency, would 482 grams of ethanol provide more, less, or the same amount of energy as 482 grams of 2- methylheptane v more less the same amountarrow_forwardConsider Cl2+F2=CLF3 (ΔH°f = -164.65 kJ/mol) . If 42 g of F2 is combined with 142 g of Cl2, how much grams of ClF3 can be produced? How much of the reagent is left over? What is the enthalpy change?arrow_forward
- Under certain circumstances, carbon dioxide, CO2(g)CO2(g), can be made to react with hydrogen gas, H2(g)H2(g), to produce methane, CH4(g)CH4(g), and water vapor, H2O(g)H2O(g): CO2(g)+4H2(g)→CH4(g)+2H2O(g) How many moles of methane are produced when 25.1 molesmoles of carbon dioxide gas react with excess hydrogen gas? Express your answer with the appropriate units. For example, write the unit moles as mol. How many moles of hydrogen gas would be needed to react with excess carbon dioxide to produce 88.1 molesmoles of water vapor? Express your answer with the appropriate units. For example, write the unit moles as mol.arrow_forwardAcrylonitrile is an important building block for synthetic fibers and plastics. Over 1.4 billion kilograms of acrylonitrile are produced in the United States each year. The compound is synthesized from propene (C3H6) in the following reaction: 2C3H6(g) + 2NH3(g) + 30₂(g) →2C3H3N(I) + 6H₂O(g) Determine how many kg of acrylonitrile can be prepared from 1480 kg of propene, 788 kg of ammonia, and 1980 kg of oxygen.arrow_forwardThe human body burns glucose (C6H₁2O6) for energy according to this chemical reaction: C6H12O6 +60₂-6CO₂ + 6H₂O The products of the reaction are carbon dioxide (CO₂) and water (H₂O). Interestingly, all of the carbon dioxide and much of the water exits the body through the lungs: on every breath, the average person exhales 500. mL of air, which is typically enriched to 4% CO₂ and 5% water vapor by volume. In short, when a person loses weight by dieting, the weight that is lost actually departs his body as a gas, every time he exhales. Each kilogram of body fat lost requires exhaling about 2.9 kg of carbon dioxide. Calculate how many breaths it takes an average person to "exhale" 1.00 kg of fat. Round your answer to the nearest thousand. You'll need to know that the density of CO₂ is 2.0 kg/m³. 0 ☐ ☐x10 Xarrow_forward
- Aqueous hydrobromic acid HBr reacts with solid sodium hydroxide NaOH to produce aqueous sodium bromide NaBr and liquid water H2O. If 10.0g of water is produced from the reaction of 58.3g of hydrobromic acid and 50.1g of sodium hydroxide, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it. I keep getting this problem wrong because of the sig. digits, dont round till the end because if it is off by 0.1, it will be incorrect.arrow_forwardConsider the reaction. heat 2 Al(s) + Fe,0,(s) Al, 0,(s) + 2 Fe(1) If 10.7 kg Al reacts with an excess of Fe, O3, how many kilograms of Al,O, will be produced? mass of Al, O, produced: kg * TOOLS x10arrow_forwardAqueous hydrobromic acid HBr reacts with solid sodium hydroxide NaOH to produce aqueous sodium bromide NaBr and liquid water H2O. If 2.35g of water is produced from the reaction of 74.4g of hydrobromic acid and 18.0g of sodium hydroxide, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY