
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
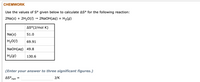
Transcribed Image Text:CHEMWORK
Use the values of S° given below to calculate AS° for the following reaction:
2Na(s) + 2H20(I) → 2NAOH(aq) + H2(g)
AS°(J/mol K)
Na(s)
51.0
H20(1)
69.91
NaOH(aq) 49.8
H2(g)
130.6
(Enter your answer to three significant figures.)
AS°,
J/K
rxn
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using only the data given below, determine AG° (in kJ) for the following reaction. NO(g) + O(g) → NO2(9) Remember that AG is a state function, just like ΔΗ. 2 03 (g) → 3 02(9) AG° = - 326 kJ 02(g) → 2 0(g) AG° = +463.6 kJ NO(g) + O3 (g) → NO2(g) + O2 (9) AG° = - 198.3 kJ kJarrow_forwardConsider the reaction 2NaOH(aq) + H2(g)—2Na(s) + 2H2O(l) for which AH° = 368.6 kJ and AS° = 15.30 J/K at 298.15 K. (1) Calculate the entropy change of the UNIVERSE when 1.759 moles of NaOH(aq) react under standard conditions at 298.15 K. ASuniverse= J/K (2) Is this reaction reactant or product favored under standard conditions? (3) If the reaction is product favored, is it enthalpy favored, entropy favored, or favored by both enthalpy and entropy? If the reaction is reactant favored, choose 'reactant favored'. Submit Answerarrow_forwardConsider the following reaction: 3C(s) + 3H2(g) → C3H6(g); AG°= 60.0 kJ and AS° =-256.7 J/K at 298 K What is the equilibrium constant and AH° at 298 K for this reaction? Express AH° in kJ and use 3 significant figures for both answers. AH° = kJ and Keq x 10arrow_forward
- Consider the reaction 6CO2(g) + 6H20((1) C,H12O6 + 602(g) for which AH° = 2.801×10° kJ and AS° = -259.0 J/K at 298.15 K. (1) Calculate the entropy change of the UNIVERSE when 1.722 moles of CO2(g) react under standard conditions at 298.15 K. ASuniverse J/K (2) Is this reaction reactant or product favored under standard conditions? (3) If the reaction is product favored, is it enthalpy favored, entropy favored, or favored by both enthalpy and entropy? If the reaction is reactant favored, choose 'reactant favored'.arrow_forwardQuestion 18 of 25 > Given the reactions, X(s) + 0,(g) –→ XO(s) AH = -562.9 kJ XCO,(s) XO(s) + CO, (g) AH = +447.1 kJ what is AH for this reaction? X(S) + 글0(g) + CO,(g) XCO,(s) ΔΗ- kJarrow_forwardPlease provide fullarrow_forward
- Given the information A+ B → 2D AH° = -682.0 kJ AS° = 296.0 J/K С — D ΔΗ. 392.0 kJ AS = -103.0 J/K calculate AG at 298 K for the reaction А +В — 2C -251.60 AG kJ Incorrectarrow_forwardUse the molar DHf° under the formulas to calculate DH°rxn for the equations as balanced: 1. 2B2H6(g) + 3CO2(g) --> 2B2O3(s) + 3CH4(g) ΔH°rxn = _______ kJ ΔH°f = +36 –394 –1274 –75 kJ/mol exo ? endo_thermic 2. 2P2O5 + 2CaC2 -->P4 + 2CaCO3 + 2CO2 ΔH°rxn = _______ kJ ΔH°f = –1505 –59 ___ –1207 –394 kJ/mol exo ? endo_thermic 3. 2Na2CrO4(s) + 10HCl(g) --> 4NaCl(s) + 3Cl2(g) + Cr2O3(s) + 5H2O(l) ΔH°f = –1342 –92 –411 ___ –1140 –286 kJ/mol ΔH°rxn = _______ kJ exo ? endo_thermicarrow_forward"M" represents a metallic element. "X" represents a nonmetallic element. Calculate ΔH for the process M3X4(s) ⟶ 3M(s) + 2X2(g) from the following information: M(s) + ½X2(g) ⟶ MX(s) ΔH°= -286.2 kJ 3MX(s) + ½X2(g) ⟶ M3X4(s) ΔH°= -84.3 kJarrow_forward
- Using the values in the table below, calculate AGxn at 375 K for the reaction: 3 CO g) + 4 NH3(g) → 3 CH3OH(g) + 2 N2(g) AH°F, kJ/mol AG°, kJ/mol S°, J/mol K COg) CH3OH(g) CH3OH NH3(aq) NH3(g) N2(9) -111 -137 198 -201 -162 238 -239 -166 127 -80 -27 111 -46 -17 193 192 +55 +15 -7 +100,414 +150arrow_forwardGiven the information AS = 302.0 J/K AS = -153.0 J/K А +В — 2D AH = 699.7 kJ С — D AH' = 398.0 kJ calculate AG at 298 K for the reaction A +B → 20 AG" = kJarrow_forwardO-234 kJ 234 kJ -150. kJ -117 kJ Given: -1835kJ AH°, AHᵒf (kJ/mol) -941 -840 QUESTION 12 Use the standard reaction enthalpies given below to determine AH°rxn for the following reaction: P4(g) + 10 Cl₂(g) → 4 PC|5(s) PC15(s) → PC13(g) + Cl₂(g) P4(g) + 6 Cl₂(g) → 4 PCl3(g) rxn IF7(g) IF 5(9) 2(g) 62.42 = ? ΔΗ° rxn AH°rxn = +157 kJ = -1207 KJ QUESTION 13 Use the AH°f and AH°rxn information provided to calculate AH°f for IF: IF7(9) + 12(g) → IF5(g) + 2 IF(g) AH°rxn = -89 kJarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY