
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:5:34
Question 13.c of 13
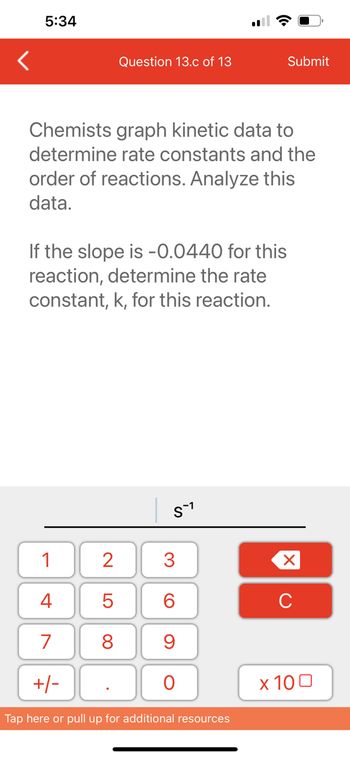
Chemists graph kinetic data to
determine rate constants and the
order of reactions. Analyze this
data.
If the slope is -0.0440 for this
reaction, determine the rate
constant, k, for this reaction.
1
4
7
+/-
2
5
8
S-1
3
60
9
O
Submit
Tap here or pull up for additional resources
XU
x 100
Expert Solution
arrow_forward
Step 1
(i) The units of rate constant for first order reaction are time-1.
In the given question, the units of rate constant are given as s-1 where s is the unit of time. So, the reaction is first order.
(ii) The integrated rate law for the first order reaction is . If a plot of ln[A] vs time (t) is a straight line then the reaction is first order with respect to "A". Here,
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The rate of a certain reaction is given by the following rate law: rate =k[N,]H, ° Use this information to answer the questions below. olo What is the reaction order in N,? x10 Ar What is the reaction order in H,? What is overall reaction order? At a certain concentration of N, and H2, the initial rate of reaction is 0.090 M / s. What would the initial rate of the reaction be if the concentration of N, were doubled? Round M S your answer to 2 significant digits. The rate of the reaction is measured to be 95.0 M / s when [N2] = 0.99 M and [H2] = 1.1 M. Calculate the value of the -2 -1 k = •S rate constant. Round your answer to 2 significant digits.arrow_forwardHow can you determine the instantaneous rate of a reaction from a given set of data and/ or the graph generated from the data?arrow_forwardWhat is an intermediate within a reaction mechanism?arrow_forward
- [H2O2 (aq)], M 0.0850 0.170 0.250 0.350 0.350 0.350 0.350 [l* (aq)], M 0.100 0.100 0.100 0.100 0.200 0.300 0.400 A [H₂O₂ (aq)] At 1.4 2.9 4.2 5.9 12 18 24 -, (M-s¯¹) x 10-4arrow_forwardWhat two quantities are needed to calculate the rate of a reaction?arrow_forwardThe rate of a certain reaction is given by the following rate law: rate =k| N,| H2 -k[N;][H+]° Use this information to answer the questions below. What is the reaction order in N,? What is the reaction order in H2? What is overall reaction order? At a certain concentration of N, and H2, the initial rate of reaction is 0.620 M / s. What would the initial rate of the reaction be if the concentration of N, were halved? Round M S your answer to 3 significant digits. The rate of the reaction is measured to be 24.0 M / s when [N2] = 1.5 M and [H2] = 0.16 M. Calculate the value of the k = |M -2 -1 •S rate constant. Round your answer to 2 significant digits.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY