
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Only typed answer with explanation otherwise leave it
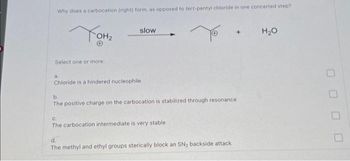
Transcribed Image Text:Why does a carbocation (right) form, as opposed to tert-pentyl chloride in one concerted step?
Тонг
Select one or more:
slow
a.
Chloride is a hindered nucleophile
b.
The positive charge on the carbocation is stabilized through resonance
C.
The carbocation intermediate is very stable
d.
The methyl and ethyl groups sterically block an SN₂ backside attack
H₂O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please help with the questionarrow_forward= teaching and lea x /ilm/takeAssignment/takeCovalentActivity.do?locator-assignment-take juju_ arch CuO chemical name - Google Se xb Answered: A 30.7 ml sample of = x + Free Online Survey... Submit Answer Home - Academia... Create Your Rubric... The Utility Experts .... [Review Topics] [References] Use the References to access important values if needed for this question. A 24.3 mL sample of a 0.318 M aqueous nitrous acid solution is titrated with a 0.405 M aqueous barium hydroxide solution. What is the pH at the start of the titration, before any barium hydroxide has been added? pH = Retry Entire Group 8 more group attempts remaining hp 16 Quando a rede soci... # 0 » NEW * 4x Carrow_forwardI only need parts d. And e. Solved please. Thanks.arrow_forward
- The volume of water needed to dissolve 0.0509 grams of lead fluoride is Assume no volume change upon addition of the solid. Submit Answer Retry Entire Group 7 more group attempts remainingarrow_forwardchemistryarrow_forwardGC 4253_L03_Chemical_Nomendature (2) - Microsoft Word rences Mailings Review View 11 A A AaBbCc[ AABB AaBbC 4. x' Aa ab T Normal Heading 1 T No Spaci nt Paragraph 5. P4O10 6. SO2 7. COarrow_forward
- Select one for each boxarrow_forwardA. Determine whether the curved arrow(s) shown below generate a valid or invalid resonance structure. Draw the resonance structure that would result from each properly drawn arrow and identify the arrow pushing pattern (i.e. allylic positive charge, allylic lone pair, pi bond between atoms of a different electronegativity, lone pair adjacent to a positive charge, alternating pi bonds in a ring.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY