
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Born-Haber cycle; I don’t know how to formulate it for this reaction.
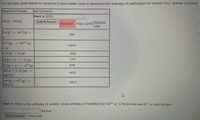
Transcribed Image Text:Use the data given below to construct a Born-Haber cycle to determine the enthalpy of sublimation for Xornon (Xo). Answer in kJ/mol.
Reaction/Process
AH°(kJ/mol)
Part 1: 5751
Xo(s)Xo(g)
Submit Answer
Previous
Incorrect. Tries 12/99
Tries
Xo(g)
Xo+(g) +
590
e
Xo+(g)
Xo2+(g)
1010
+ e
2 0(g) 02(g)
-498
O(g) + er –
-141
O (g) + e- -→
o2 (g)
878
Xo(s) + V2 02(g)
Xo0(s)
-635
Xo (g) + 02 (g)
XoO(s)
-3414
Part 2: What is the enthalpy of solution given enthalpy of hydration for Xo²+ is -1754 kJ/mol and 02- is -933 kJ/mol?
kJ/mol
Submit Answer
Tries 0/99
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete these reactionsarrow_forwardGive detailed Solution with explanation needed of each reaction....don't give Handwritten answerarrow_forward(a) In an acid-catalyzed hydration, one of the following 10 carbon alkynes is expected to produce a single hydration product? Select the correct alkyne and draw the structure of the hydration product that is formed from this alkyne. (I) 2-decyne; (II) 3-decyne; (III) 4-decyne; (IV) 5-decyne; (V) none of these will give a single hydration product. (b) The reaction shown below gives Compound X as the major product. The mass spectrum of X is shown. Br2, H20 Compound X 100 - MS-IW-5644 80 60 40 - 20 - 20 40 60 80 100 120 140 160 180 200 220 m/z Considering the reactions of alkynes and the MS data, de duce which of following structures corresponds to X: Br Br HO, IV V I II II Support your answer with a reaction mechanism for fomation of X and identification of relevant peaks in the mass spectrum. 12 Relative Intensityarrow_forward
- 6- In a 1H-NMR spectra on a scale of 0 – 10 ppm, Show the exact locations in ppm of 1-the saturated hydrocarbons, 2- the unsaturated hydrocarbons, 3- the halogenatedhydrocarbons and 4- the aromatic hydrocarbons.arrow_forwardThe two alkenes below react with HI at different rates. CH3CH=CHCH3 and CH₂=CH₂ Draw the structural formula of the MAJOR product formed by the alkene having the HIGHER reaction rate. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. TAYY [ ] در ChemDoodle Ⓡarrow_forwardStep 6: Now that you have determined the substrates and mechanism of a Diels-Alder reaction, you will learn how to recognize when you should use the Diels-Alder reaction. In a synthesis reaction, if you are given a cyclohexene product with no other obvious functional group transformations and an electron-withdrawing group two carbons away from the alkene, it is likely made via the Diels-Alder reaction. Deduce the structures of the starting materials to form the Diels-Alder adduct shown. ..... CN CN Diene + Dienophilearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY