
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
balance the following chemical equation (if necessary)
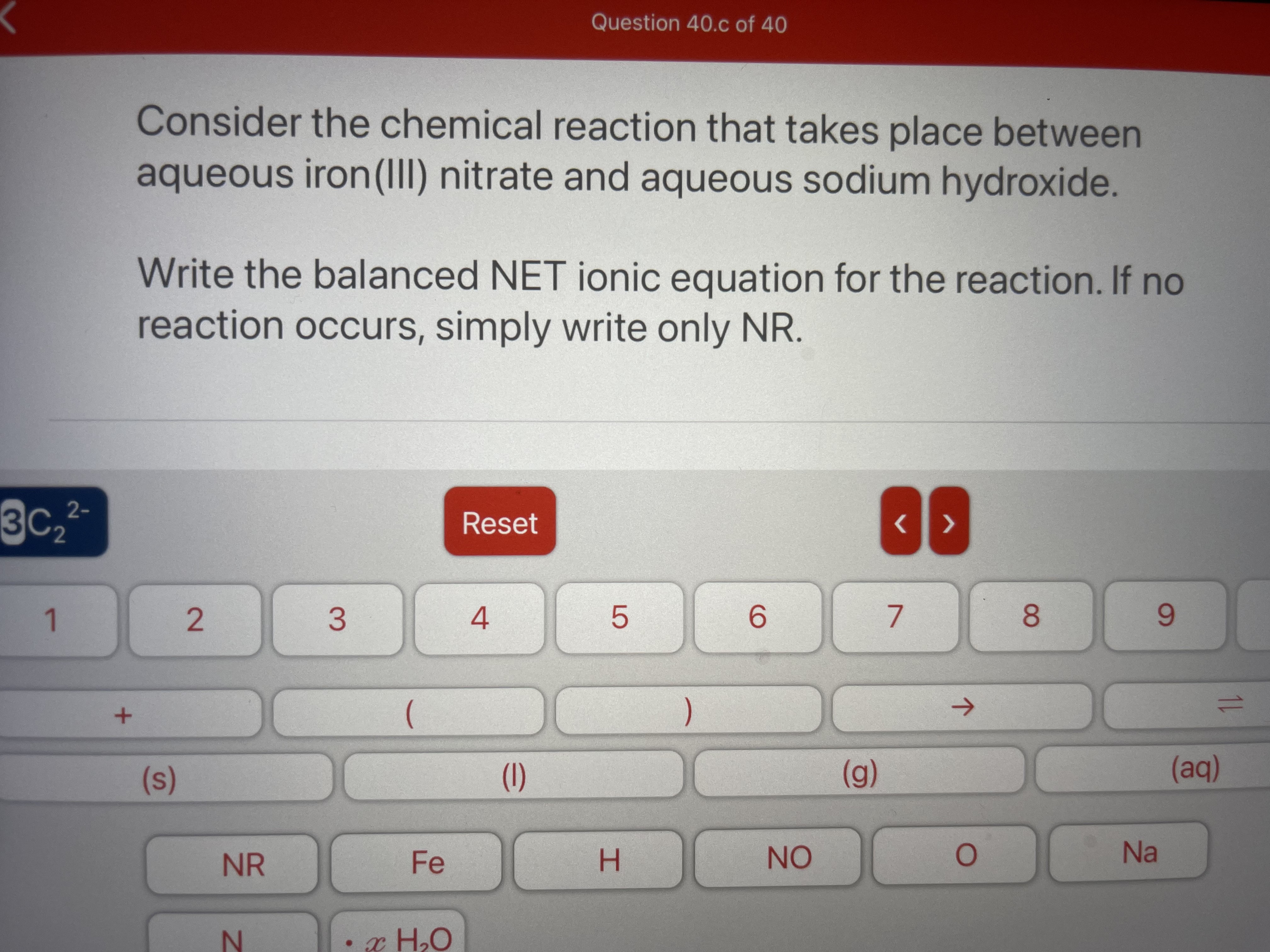
Transcribed Image Text:Question 40.c of 40
Consider the chemical reaction that takes place between
aqueous iron(II) nitrate and aqueous sodium hydroxide.
Write the balanced NET ionic equation for the reaction. If no
reaction occurs, simply write only NR.
2-
Reset
1
2.
4
6.
8.
6.
)
->
(s)
(1)
(g)
(aq)
NR
Fe
H.
NO
Na
• x H,0
1L
3.
SI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- > Combustion of hydrocarbons such as butane (C4H10) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of gaseous butane into gaseous carbon dioxide and gaseous water. 8 2. Suppose 0.280 kg of butane are burned in air at a pressure of exactly 1 atm and a temperature of 13.0 °C. Calculate the volume of carbon dioxide gas that is produced. Round your answer to 3 significant digits. OL Explanation Check Deconved 8 X x10 S ? ▷ olo Ar BH Terms of Use | Privacy Center | Accessibility Wa Pr Q:arrow_forwardAqueous iron (III)chloride reacts with aqueous sodium hydroxide to form solid iron (III) hydroxide and another aqueous product. Write a full balanced equation for the above reaction statement.arrow_forwardPredict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced and contains state symbols after every reactant and product. HNO, (ag) + H,0 (1) → D ローロarrow_forward
- Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.' Write a chemical equation and balance them.arrow_forwardCombustion of hydrocarbons such as dodecane (C₁2H26) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid dodecane into gaseous carbon dioxide and gaseous water. 00 2. Suppose 0.290 kg of dodecane are burned in air at a pressure of exactly 1 atm and a temperature of 12.0 °C. Calculate the volume of carbon dioxide gas that is produced. Be sure your answer has the correct number of significant digits. OL x10 Ś ? 00. 18 Ararrow_forwardCombustion of hydrocarbons such as heptane (C,H,6) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid heptane into gaseous carbon dioxide and gaseous water. x10 2. Suppose 0.480 kg of heptane are burned in air at a pre of exactly 1 atm and a temperature of 15.0 °C. Calculate the volume of carbon dioxide gas that is produced. Be sure your answer has the correct number of significant digits.arrow_forward
- Nitrogen trioxide gas and nitrogen dioxide gas combine to produce dinitrogen pentoxide gas . Write a balanced chemical equation for this reaction.arrow_forwardCombustion of hydrocarbons such as dodecane (C1,H26) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid dodecane into gaseous carbon dioxide and gaseous water. x10 2. Suppose 0.450 kg of dodecane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 °C. Calculate the volume of carbon dioxide gas that is produced. Be sure your answer has the correct number of significant digits. Larrow_forwardBalance chemical equation H2SO4+NaOH---->arrow_forward
- Balance the equation: solid magnesium chloride and aqueous potassium phosphate react to produce aqueous potassium chloride and solid magnesium phosphate.arrow_forwardCombustion of hydrocarbons such as dodecane (C1,H26) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid dodecane into gaseous carbon dioxide and gaseous water. O-0 Ox10 2. Suppose 0.140 kg of dodecane are burned in air at a pressure of exactly 1 atm and a temperature of 12.0 °C. Calculate the volume of carbon dioxide gas that is produced. Round your answer to 3 significant digits.arrow_forwardBalance the chemical equation below using the smallest possible whole number stoichiometric coefficients. Ca, (PO,), (s) + SiO, (s) + C(s) → CaSio, (s) + P, (s) + CO(g) 2 alo Ararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY