
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
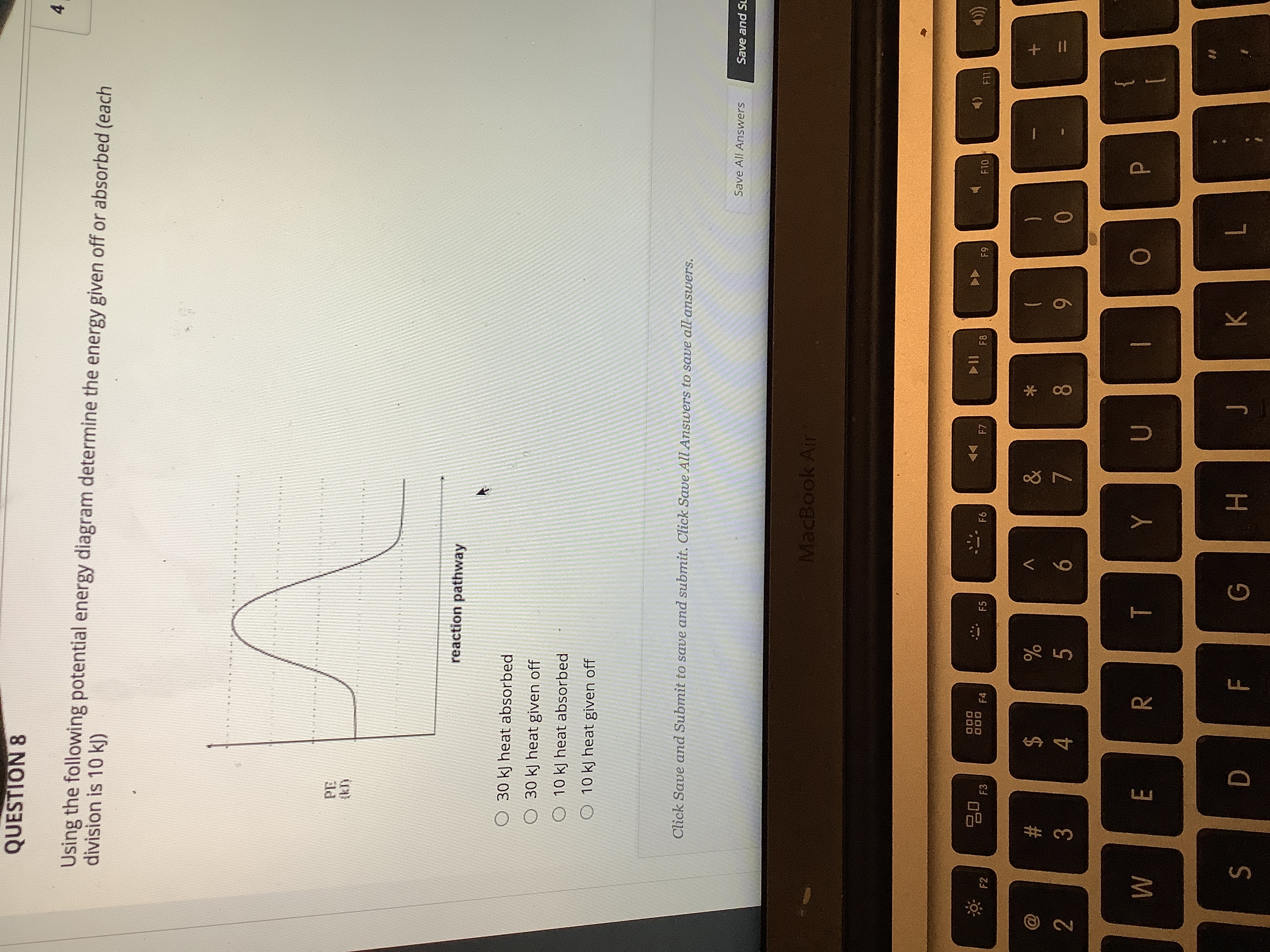
Using the following potential energy diagram determine the energy given off or absorbed (each division is 10kj)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- these are 2 different questions please label as 1 and 2arrow_forwardWhen 1.42 g of iron reacts with 1.80 g of chlorine, 3.22 g of iron(II) chloride (FeCl2, molar mass = 126.75 g/mol) and 6.8 kJ of heat is produced. What is the enthalpy change for the reaction when 1 mole of iron(II) chloride is produced? round to sig figsarrow_forwardI need to find to what equals to 320 J and I’m kinda stuck on this.arrow_forward
- I need some help solving this questionarrow_forwardIf 0.24 mols of substance B is able to produce 137J of heat. What is the change in reaction enthalpy in J associated with the following balanced reaction. (Hint: *pay attention the the coefficient for substances B *determine the sign for heat, is it + or -). Round and report your answer to an integer without decimal place including + or- - sign. Only enter numeric value, no unit. A+2B → AB2 + heat AHren =???arrow_forwardA bicyclist is stopped at the entrance to a valley, as sketched below: B D Where would the bicyclist have the highest potential energy? Where would the bicyclist have the lowest potential energy? Where would the bicyclist have the highest kinetic energy? Where would the bicyclist have the highest speed? Would the bicyclist's kinetic energy be higher at C or B? Would the bicyclist's potential energy be higher at C or B? Would the bicyclist's total energy be higher at C or B? Suppose the bicyclist lets off the brakes and coasts down into the valley without pedaling. Even if there is no friction or air resistance to slow her down, what is the farthest point the bicyclist could reach without pedaling? E (choose one) ✓ (choose one) (choose one) ✓ (choose one) ✓ (choose one) (choose one) (choose one) X (choose one) Ś ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY