
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How many moles of NaOH did it take to reach the equivalence point ?
what is shown in the pictures is all I was given.
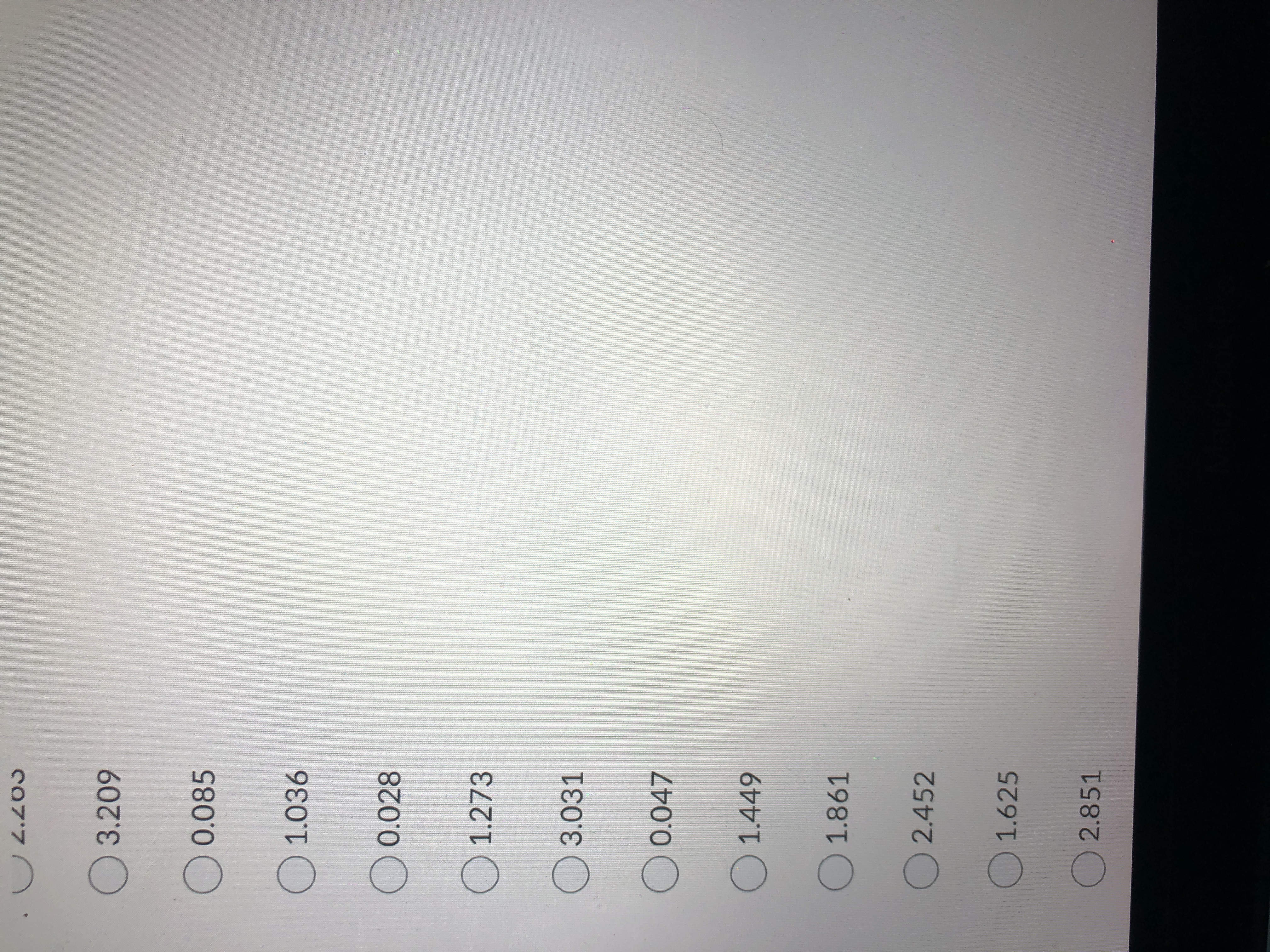
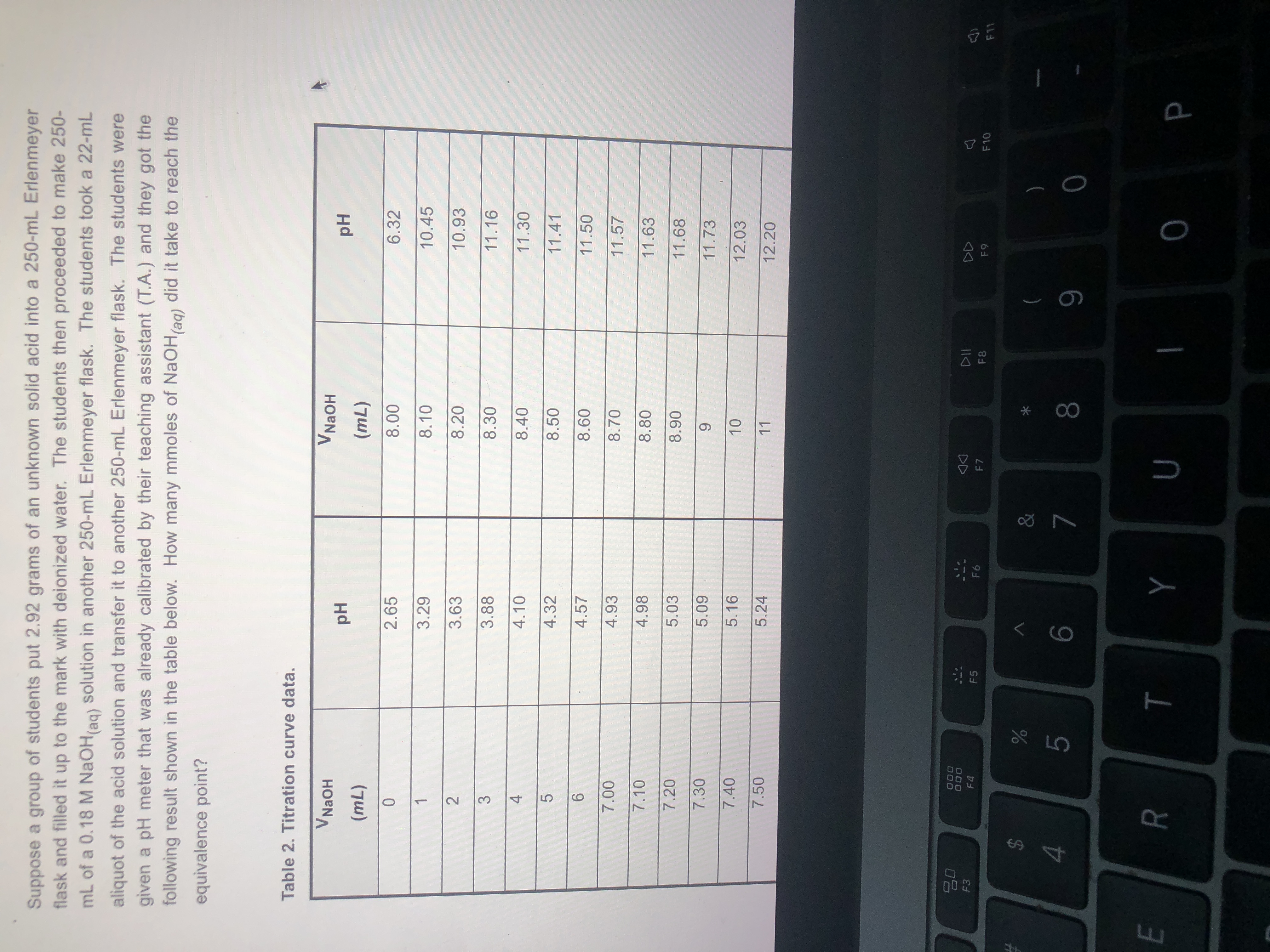
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2 Na2CO3+Pb(OH)4= Pb(CO3)2 + 4 NaOH TOTAL IONIC EQN: net iomice eqn:arrow_forwardCalculate the pH of a solution in which one normal adult dose of aspirin (670 mg ) is dissolved in 10 ounces of water. Express your answer to one decimal place. pH = %3Darrow_forwardWhat is the [OH'] of a solution with a [H*] of 5.8 x 10-¹2? the answer is not listed 2.3 x 10:¹¹ M 11.23 2.3 x 10³ M 0.0017 M 3.54arrow_forward
- How do you find Keq if given an equation and Ka and Kb? EX: Someone found a smashed box of smelling salts in a storage closet of Fauver Stadium. The field hockey team manager, being a chemistry pro, knew that if she could convert all of the ammonia (NH3) found in the smelling salts into an ammonium salt (NH4+ + X), she could get rid of the horrid smell. If the closet contained 100 mL of 1.0 M NH3 solution and the field hockey team manager/chemistry pro added 100 mL of a 1.0 M solution of H2SO4. Kb for NH3 = 1.73 • 10-6, Ka for H2SO4 = 1000, Ka for HSO4-= 1.2 • 10-2 a. Provide a keq value for each of the following... H2SO4 + NH3 =HSO4 + NH4 HSO4 + NH3=SO4+ NH4 NH3 + H20= OH- + NH4 NH4 + H20= NH3 + H30 SO4 + H20= HSO4 + OH-arrow_forwardHO -OH Oxalic Acid, H₂C₂O₁ PK₁₁ = 1.27 pka2 = 4.266 H₂Ox→ HOX → Ox ²- H₂A → HA → A²- pH = ? Adding 0.20 mole of NaOH to 0.40 mole of NaHC₂O4 (s) with total volume of 500 mL. pH = ? 150 g of NaHC₂O4 (s) dissolved in DI water total volume of 500 mL. pH = ? Adding 0.20 mole of HCI to 0.40 mole of NaHC₂O4 (s) with total volume of 500 mL. pH = ? 0.40 Adding 0.20 mole of HCI mole of Na₂C₂O4 (s) with total volume of 500 mL. pH = ? Adding 0.40 mole of HCI to 0.40 mole of Na₂C₂O4 (s) with total volume of 500 mL. pH = ? Important Key Concept Exercisearrow_forwardWhat is the ratio of bicarbonate ion to carbonic acid ([HCO−3]/[H2CO3])([HCO3−]/[H2CO3]) in blood serum that has a pHpH of 7.40? Express your answer using three significant figures.arrow_forward
- The concentration of H+ in a glass of milk is around 3.2 x 10-7 The pH must then be around (Round to the 2nd decimal)arrow_forwardHow many moles of the following molecule are needed to neutralize 2 moles of calcium hydroxide? H–C-C-C C- H. H H H-0-Iarrow_forwardWhich of the following (assuming the same concentration in water) would make a solution with the lowest hydrogen ion concentration? Group of answer choices H2S (Ka= 8.9×10−8) NH4+ (Ka= 5.6 × 10−10) NH3 ( Kb= 1.8 × 10−5) HC2H3O2 (Ka= 1.8×10−5) HClarrow_forward
- Mix equal parts acetic acid (CH3COOH) and sodium acetate (NaCH3COO). Add HCl. Write chemical reaction that took place with added HCl.arrow_forwardSome chemical compounds are listed in the first column of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. The important chemical species that would be present in this solution are written in the second column of the table. Use the checkboxes to classify each compound. compound HCI W Ba (OH), HC,H,O, KCI Explanation patric(most common) 1 Other place . с important species present when dissolved in water E н,о, ст. н.о Ba, OH, H₂O но, с.н,о.. нс.н, о,, H,о K, CI, H₂O Check $ 4 R type of compound (check all that apply) ionic molecular strong weak strong weak acid acid base base Speciation is happening because the two groups split and live in other places 0 % 5 0 0 0 0 0 T 0 0 0 G Search or type URL 0 0 0 0 X Y 0 0 0 0 S & 0 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Access 0.5 U * 00 8 9arrow_forward2. HPO42taq) + H2Ou) = H2PO4°(aq) + OH 3. CH3COOH(aq) + H2O) = CH3COOH2*(aq) + OH(aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY