Table 2. Exercise on electronic and molecular geometrics for the lab report). Type of Exempl Number Namber Molecule of of Non Bonding conding Electron Electron 16 32 2885 18 15 22 18 CO₂ CB₂ H₂O IBT, ICI, (TCL) # Sef Wemains Douraine O || 8 4 2 12 3 Electron Geometry Linear Tetrahedral Tetrahedral Molecular Geometry isnore electrons Linear - Ben Linear Pelar or Nonpolar non-Polar Polar #The noga ve charge on the anions adds one clectron to the valonco c'octrons, accounting for a tou 35 valencs electrons
Table 2. Exercise on electronic and molecular geometrics for the lab report). Type of Exempl Number Namber Molecule of of Non Bonding conding Electron Electron 16 32 2885 18 15 22 18 CO₂ CB₂ H₂O IBT, ICI, (TCL) # Sef Wemains Douraine O || 8 4 2 12 3 Electron Geometry Linear Tetrahedral Tetrahedral Molecular Geometry isnore electrons Linear - Ben Linear Pelar or Nonpolar non-Polar Polar #The noga ve charge on the anions adds one clectron to the valonco c'octrons, accounting for a tou 35 valencs electrons
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
Our experts are unable to provide you with a solution at this time. Try rewording your question, and make sure to submit one question at a time. We've credited a question to your account.
Your Question:
please solve correctly
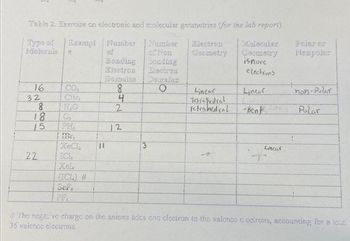
Transcribed Image Text:Table 2. Exercise on electronic and molecular geometrics for the lab report).
Type of Exempl Number
Namber
Molecule
of
of Non
Bonding
conding
Electron Electron
16
32
2885
18
15
22
18
CO₂
CB₂
H₂O
IBT,
ICI,
(TCL) #
Sef
Wemains Douraine
O
||
8
4
2
12
3
Electron
Geometry
Linear
Tetrahedral
Tetrahedral
Molecular
Geometry
isnore
electrons
Linear
- Ben
Linear
Pelar or
Nonpolar
non-Polar
Polar
#The noga ve charge on the anions adds one clectron to the valonco c'octrons, accounting for a tou
35 valencs electrons
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
