
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Need help with these two questions
Transcribed Image Text:言
云
7.
Tools
Window
Help
Thu Oct 28 9:33 A
c163_5_CA21_f20 Titration Curves TAV v1.0.pdf
Page 1 of 3
Q Search
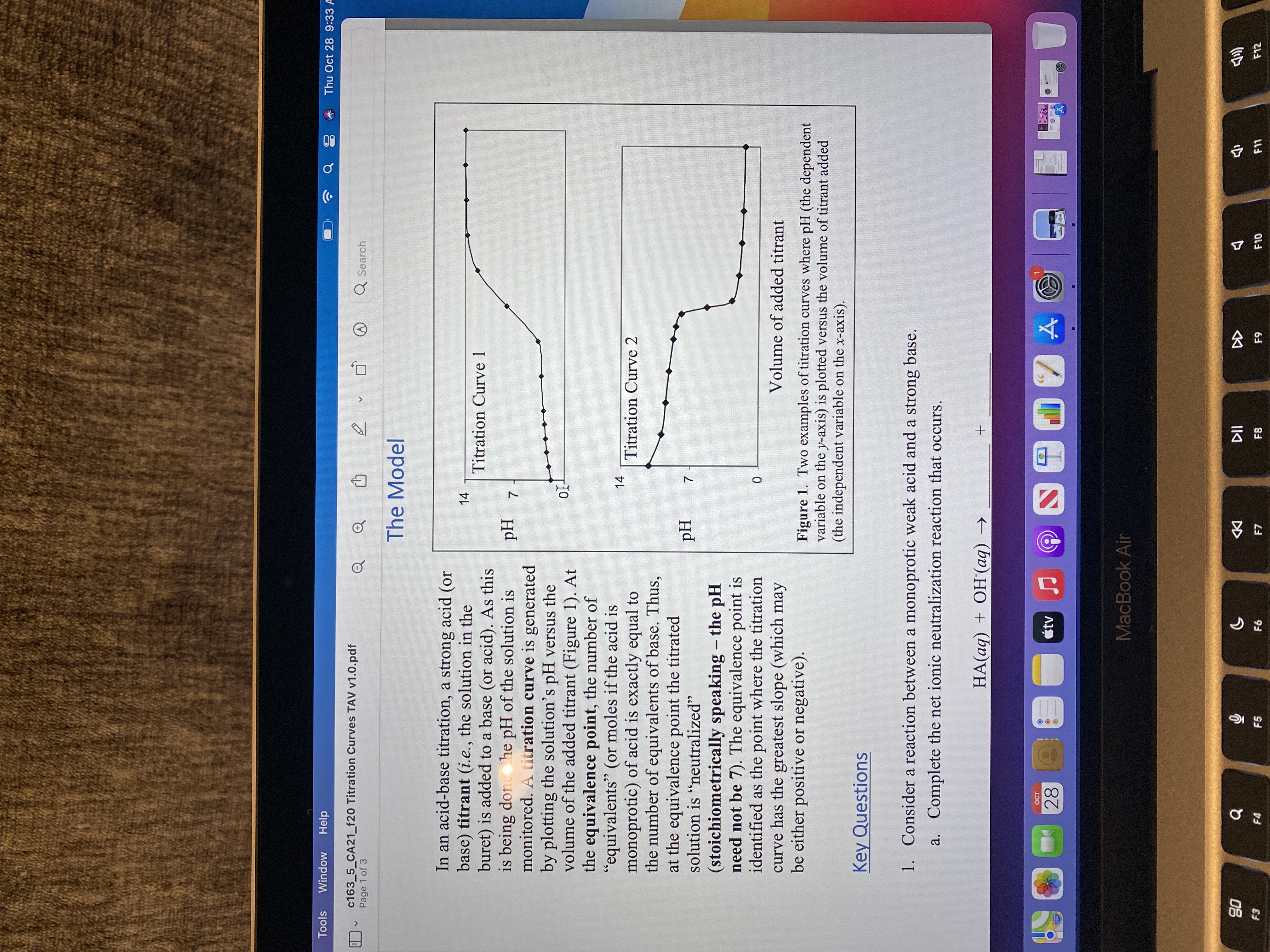
The Model
In an acid-base titration, a strong acid (or
base) titrant (i.e., the solution in the
buret) is added to a base (or acid). As this
is being done he pH of the solution is
monitored. A titration curve is generated
by plotting the solution's pH versus the
volume of the added titrant (Figure 1). At
the equivalence point, the number of
"equivalents" (or moles if the acid is
monoprotic) of acid is exactly equal to
the number of equivalents of base. Thus,
at the equivalence point the titrated
solution is "neutralized"
14
Titration Curve 1
Z Hd
66
14
Titration Curve 2
Hd
(stoichiometrically speaking - the pH
need not be 7). The equivalence point is
identified as the point where the titration
curve has the greatest slope (which may
be either positive or negative).
|
Volume of added titrant
Figure 1. Two examples of titration curves where pH (the dependent
variable on the y-axis) is plotted versus the volume of titrant added
(the independent variable on the x-axis).
Key Questions
1. Consider a reaction between a monoprotic weak acid and a strong base.
a. Complete the net ionic neutralization reaction that occurs.
HA(aq) + OH(aq) →
OCT
28
MacBook Air
DD
F7
08
F4
F5
F8
F10
F11
F12
63
Transcribed Image Text:会。
* 00
ols
Window
Help
Thu Oct 28
c163_5_CA21_f20 Titration Curves TAV v1.0.pdf
Page 1 of 3
Q Search
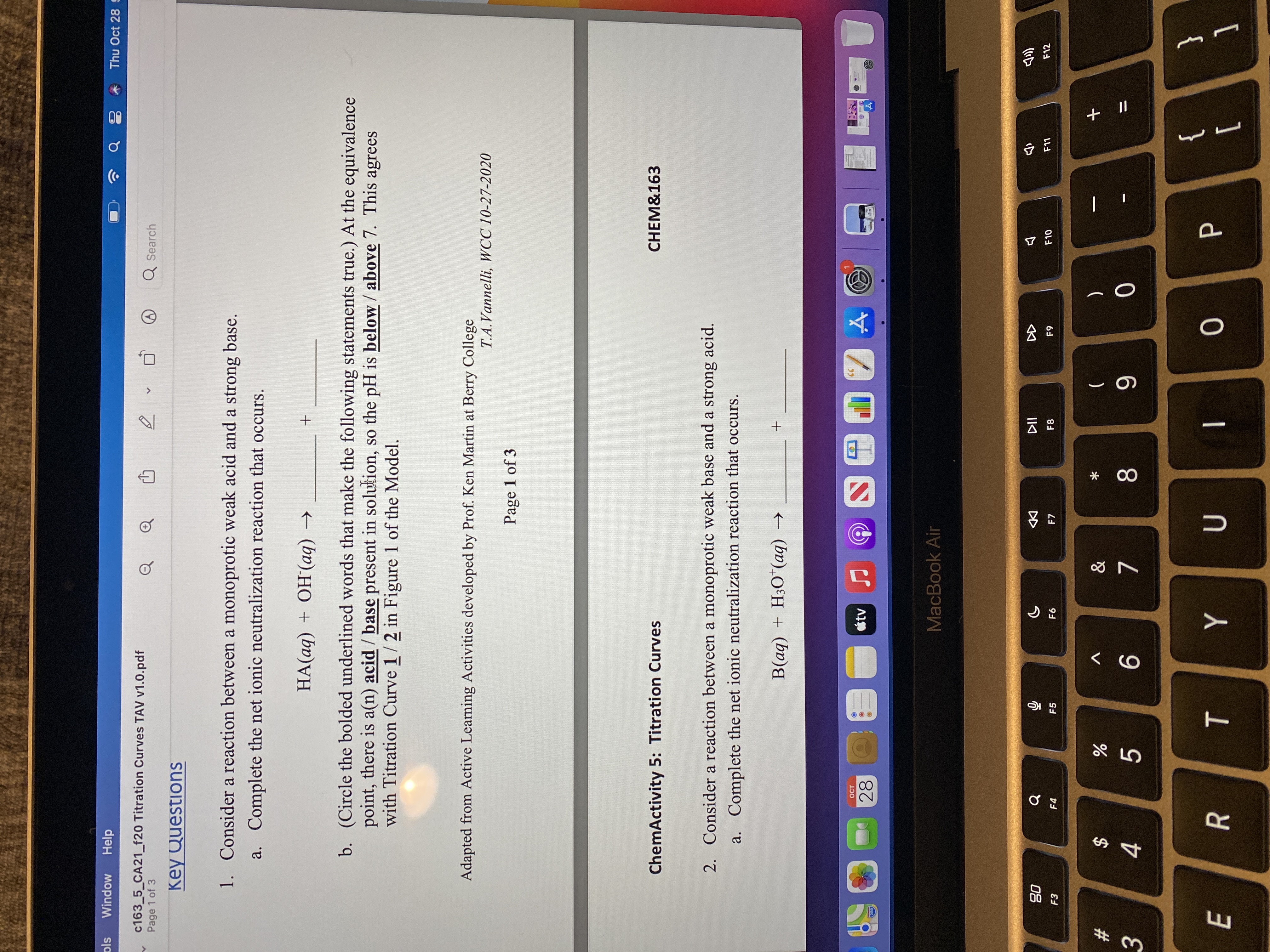
Key Questions
1. Consider a reaction between a monoprotic weak acid and a strong base.
a. Complete the net ionic neutralization reaction that occurs.
HA(aq) + OH(aq) →
b. (Circle the bolded underlined words that make the following statements true.) At the equivalence
point, there is a(n) acid / base present in solution, so the pH is below / above 7. This agrees
with Titration Curve 1/2 in Figure 1 of the Model.
Adapted from Active Learning Activities developed by Prof. Ken Martin at Berry College
T.A.Vannelli, WCC 10-27-2020
Page 1 of 3
CHEM&163
ChemActivity 5: Titration Curves
2. Consider a reaction between a monoprotic weak base and a strong acid.
a. Complete the net ionic neutralization reaction that occurs.
+ (bv),O°H + (bv
OCT
28
MacBook Air
08
F3
币
F5
DD
F7
F12
F10
F8
F4
&
(
V
一
23
$
8.
6
3.
}
1
{
[
R
|
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- QUESTION 4 Neurotransmitters produce their effects more slowly quickly QUESTION 5 compared to hormones. If calcium ions are removed from the fluid surrounding a pre-synaptic neuron, then the effects of that neuron's neurotransmitter will be: enhanced diminishedarrow_forwardCan you please solve this question and say the correct letterarrow_forwardQuestion 26 A new class of drug has been developed for patients with hyperglycemia type 2 diabetes; it works by acting as an agonist for the GLP-1 receptor, which reduces blood glucose levels. How does this new drug reduce the concentration of blood glucose? O A. Decreasing renal reabsorption of glucose OB. Long-loop negative feedback of ACTH OC. Increasing insulin release OD. Decreasing glucagon release OE. Inhibiting cortisol synthesisarrow_forward
- Question 9 A client living with schizophrenia was recently started on Risperidone, an atypical antipsychotic. What common side effects should the RN include in their health teaching? Question 9 options: Extrapyramidal side effects Neuroleptic malignant syndrome Weight gain Orthostatic hypotensionarrow_forwardStarted: Dec 9 at 8:05pm Quiz Instructions Question 29 Neurotransmitter patterns associated with depression include: O Decreased serotonin and elevated dopamine and norepinephrine O Decreased dopamine and elevated serotonin and norepinephrine O Elevated dopamine, norepinephrine and serotonin O Decreased dopamine, norepinephrine and serotonin Previousarrow_forwardQUESTION 32 Which of the following statements is not correct? AREA L CELLS A BLOOD AREA K VESSELS B 2 BLOOD VESSEL C BLOOD VESSELS D AREA M AREA E BLOOD CELLS J VESSELS F AREA G BLOOD VESSEL H AREA E is the neurohypophysis The hypothalamus is labeled AREA L Antidiuretic enters the blood at BLOOD VESSELS B AREA G is the anterior pituitaryarrow_forward
- Question 17 Prolactin is produced by the posterior pituitary hypothalamus mammary gland anterior pituitary 0000 Moving to another question will save this response. 80 F3 F4 MacBook Airarrow_forwardQuestion 22 The client with Crohn’s disease may also have the following health issue: Question 22 options: Low hemoglobin due to bloody stools Bruising due to vitamin deficiencies Periods of constipation between flare ups Significant increase risk of colorectal cancerarrow_forwardQuestion 5 The nurse discovers a client receiving warfarin is bleeding. What drug should the nurse prepare to counteract this drug? OA Vitamin E B. Protamine Sulfate C. Vitamin K D. Calcium gluconate A Moving to another question will save this response.arrow_forward
- Need help with the following question.arrow_forwardQuestion 16 A mother is asking which treatment will be most effective for her 15 year old daughter who is experiencing depression. The nurse explains which of the following treatments have been effective for individuals with depression. Question 16 options: Tricyclic Antidepressants and Electroconvulsive Therapy Selective Serotonin Reuptake Inhibitors and Cognitive Behavioural Therapy Monoamine Oxidase Inhibitors and Exercise Therapy Benzodiazepines and Problem Solving Therapyarrow_forwardI need help please.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON