
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
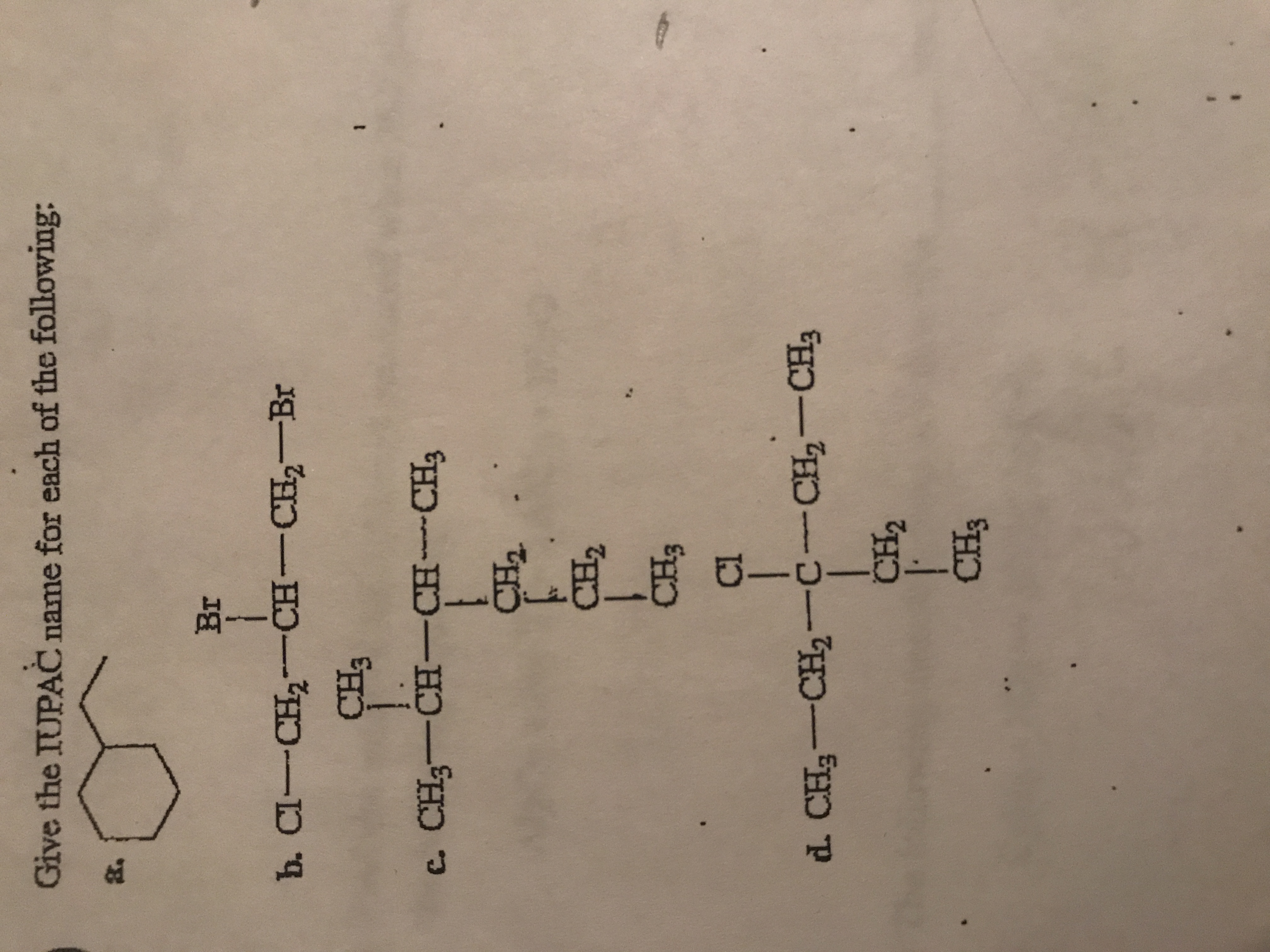
Transcribed Image Text:**Title: IUPAC Naming of Organic Compounds**
**Introduction:**
This section provides the IUPAC naming for a series of organic compounds. Understanding the nomenclature helps in identifying the structure and characteristics of chemical compounds.
**Exercises:**
**a.**
Structure: A cyclohexane ring with a propyl group attached.
**b.**
Structure: Cl-CH₂-CH(Br)-CH₂-Br
Description: The central carbon is bonded to one chlorine (Cl) and one bromine (Br), making it a 1,2-dihalide with terminal bromine.
**c.**
Structure:
- CH₃-CH(CH₂-CH₃)-CH(CH₃)-CH₂-CH₃
- A chlorine (Cl) is on the central carbon making it branched and includes a central carbon with an ethyl and methyl group attached.
**d.**
Structure:
- CH₃-CH₂-C(CH₂-CH₃)(Cl)-CH₂-CH₃
- Contains a chlorine (Cl) and an isopropyl group on a central carbon in the main chain.
**Conclusion:**
These exercises require understanding of structural formulas to correctly identify and name each compound according to IUPAC conventions. Focus on substituents and configurations provides insight into systematic naming.
Expert Solution
arrow_forward
Step 1
Since you have posted a question of multiple subparts, we can answer only first three subparts. To get the remaining subparts solved repost the question and mention this part.
A systematic name is given to an organic compound by IUPAC nomenclature. The set of rules which are used to name any compound are:
Step by stepSolved in 5 steps

Knowledge Booster
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY