
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
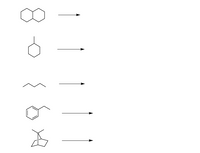
For each compound, predict the major product(s) of free-radical bromination.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Organic Chemistryarrow_forward4. Show the mechanism and predict the major products of the following reactions. Include stereochemistry where appropriate. a) HBr ROORarrow_forwardWrite the mechanism of the following bromination reaction and explain the regioselectivity of the bromination reaction (draw the mechanism, transition state, energy diagram, etc.). (Note: ‘Markovnikov’s or anti-Markovnikov’s rule’ is not a correct answer.)arrow_forward
- Bromination of an alkene by N-bromosuccinimide (NBS) in the presence of light or peroxide is a radical reaction and produces an allylic bromide. For the given bromination of 3-methylcyclopentene, select the allylic bromides from the set at the right that would be products of the reaction. CH3 CH3 CH2 CH3 CH3 Br. NBS Br Br hy or ROOR C D A Br Br- CH3 CH3 CH2 Br- E F Brarrow_forwardHow many monobromination products would be obtained from radical bromination of methylcyclohexane?arrow_forwardThe following photochemical bromination reaction could give many monobrominated products. Ignoring stereochemistry, how many different monobrominated products are theoretically possible? However, radical bromination is quite selective, so there will be one major product. Draw the major product. Ignore stereoisomers. H₂C H₂C H₂C CH₂ CH₂ Br₂ hvarrow_forward
- Predict in order of reactivity (1 being the slowest, 3 being the fastest) of primary, secondary, and tertiary alkyl halides toward nucleophilic displacement by an SN2 reaction mechanismarrow_forwardOutline how one might achieve the following transformation, showing reagents and the isolated intermediates in the synthetic scheme.arrow_forwardFor this mechanism, why are there two E1 major products? Are both of the E1 products of equal stability? Why or why not? What is the relative stability of the cycloalkenes with methyl? Also, why doesn't SN1 have two products? Is it because shifting the hydride would make it less stable?arrow_forward
- What two sets of a conjugated diene and a dienophile could be used to prepare the following compound?arrow_forwardArrange the following radicals in order of decreasing rate of bromination. UHT NOM EUT DEWarrow_forwardIntramolecular Diels-Alder reactions are possible when a substrate contains both a 1,3-diene and a dienophile, as shown in the following general reaction. , two new rings With this in mind, draw the product of the following intramolecular Diels-Alder reaction. draw structure .arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY