
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
![**Question 5**
**Predict the product for the following reactions:**
1. Starting with phenol (\( \text{C}_6\text{H}_5\text{OH} \)), it reacts with sodium hydroxide (\(\text{NaOH}\)) followed by bromopropane (\(\text{CH}_3\text{CH}_2\text{Br}\)).
2. A compound with the structure:
\[
\begin{array}{c}
\text{Phenyl acetate (C}_6\text{H}_5\text{OC(O)CH}_3)
\end{array}
\]
is treated with hydrobromic acid (\(\text{HBr}\)).
3. An ether with the following structure:
\[
\begin{array}{c}
\text{O-CH}_2\text{CH}_2\text{CH}_2\text{C}_6\text{H}_5
\end{array}
\]
undergoes a reaction when heated at 250°C.
4. An epoxide, specifically ethylene oxide (\( \text{H}_2\text{C}-\text{O}-\text{CH}_2 \)), reacts with hydrochloric acid (\(\text{HCl}\)).
Each part of the question involves predicting the resulting chemical products of these reactions given the specified reactants and conditions.](https://content.bartleby.com/qna-images/question/fb08cac0-e859-4f5b-8fac-b6815466af98/74cffd5c-9cff-4763-8e77-d01fd921c2d7/r0rwz5l_thumbnail.png)
Transcribed Image Text:**Question 5**
**Predict the product for the following reactions:**
1. Starting with phenol (\( \text{C}_6\text{H}_5\text{OH} \)), it reacts with sodium hydroxide (\(\text{NaOH}\)) followed by bromopropane (\(\text{CH}_3\text{CH}_2\text{Br}\)).
2. A compound with the structure:
\[
\begin{array}{c}
\text{Phenyl acetate (C}_6\text{H}_5\text{OC(O)CH}_3)
\end{array}
\]
is treated with hydrobromic acid (\(\text{HBr}\)).
3. An ether with the following structure:
\[
\begin{array}{c}
\text{O-CH}_2\text{CH}_2\text{CH}_2\text{C}_6\text{H}_5
\end{array}
\]
undergoes a reaction when heated at 250°C.
4. An epoxide, specifically ethylene oxide (\( \text{H}_2\text{C}-\text{O}-\text{CH}_2 \)), reacts with hydrochloric acid (\(\text{HCl}\)).
Each part of the question involves predicting the resulting chemical products of these reactions given the specified reactants and conditions.
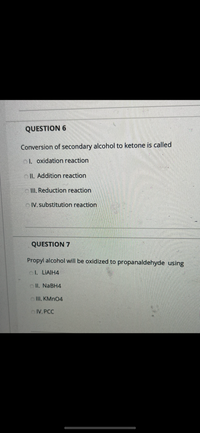
Transcribed Image Text:### Question 6
**Conversion of secondary alcohol to ketone is called:**
- I. Oxidation reaction
- II. Addition reaction
- III. Reduction reaction
- IV. Substitution reaction
---
### Question 7
**Propyl alcohol will be oxidized to propanaldehyde using:**
- I. LiAlH₄
- II. NaBH₄
- III. KMnO₄
- IV. PCC
---
These questions pertain to chemical reactions, specifically focusing on the processes of oxidation in organic chemistry.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following is the major product of the reaction shown below? * H 1. CH,MgBr, diethyl ether 2. H,O* CH,CH,CH3 CH3 H f-CH3 -CH,CH,CH3 OH --CH,CH,CH3 HO OH CH3 --H- -H --CH,CH,CH3 -CH3 CH,CH,CH3 HOarrow_forwardQuestion 32 Select the major product of the reaction sequence below. O CH3 O CH, CCHCHCH,CCH, A CN CH,CH=CHCCHCH, %3D 1. NaOEVEtOH CN CH,CH=CHC O CH, O CH,CCHCHCH,CCHCCH, C CO2H 2. H3O* CH,CH=CH D CN C ABarrow_forwardO A Question 16 CH3 1. ВН, 2. Н.О, Он The product of the reaction above is: CH3 CH3 OH OH CH3 CH3 OH OH C B O D ов O A D.arrow_forward
- Question 10 of 20 > Given two sets of reaction conditions and two products, select the structure of the common starting material. O Br O Br Br Br The unknown starting material is: `ОН OH `ОН Br₂ (2 equiv.) OH Starting material O H₂ (2 equiv.) Raney Ni OH OH OHarrow_forwardChoose the correct product!arrow_forwardWhat are the major products of these reactions?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY