
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
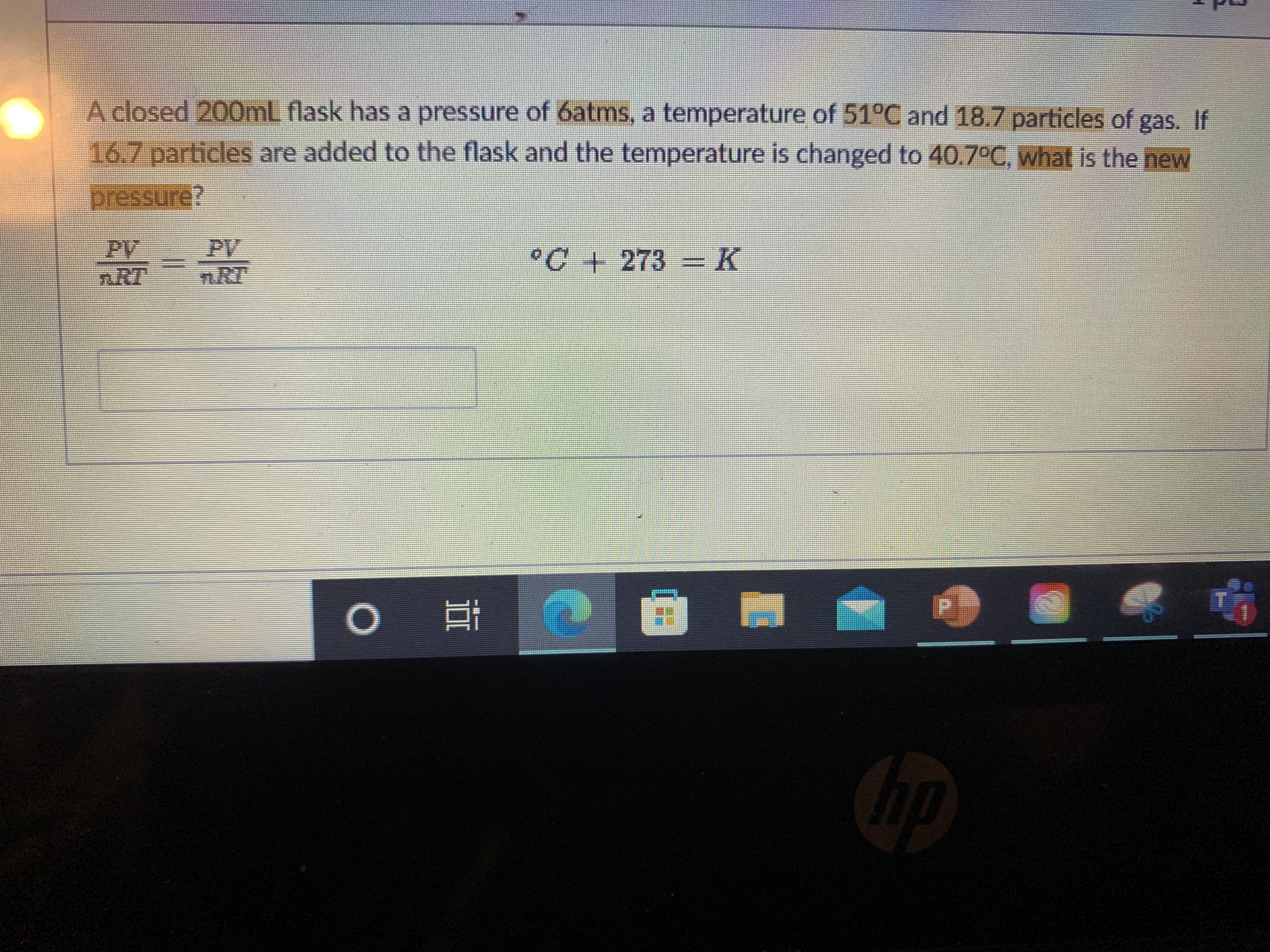
Transcribed Image Text:A closed 200mL flask has a pressure of 6atms, a temperature of 51°C and 18.7 particles of gas. If
16.7 particles are added to the flask and the temperature is changed to 40.7°C, what is the new
pressure?
PV
nRT
PV
n.RT
°C+ 273 =K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CHEM 1 Please solve correctly and give the complete solution.arrow_forwardUsing the ideal gas law (PV=nRT), calculate the temperature of a gas in a canister 10.4 L in volume. The pressure is 234,567 Pa and there are 32 moles. (R= 0.0821 L atm/ mol K) (101,325 Pa/1 atm).arrow_forwardYou inflate a balloon with 2.0 L air at a temperature of 10°C and then increase the temperature to 45°C. You noticed that the volume of the balloon has changed. Assuming the pressure and moles of gas remains constant; calculate the “new” volume, in Liters, of the balloon at 45°C. Given: V1/T1 = V2/T2, K = 273 +°C A. 2.8 L B.2.3 L C.9.0L D.0.90 Larrow_forward
- Hot gaes Expanding A mixture hot gases expands, changing volume from 2.45 L to 6.82 L, against a constant pressure of 3.22 atm. The P-Vwork involved is Latm .arrow_forwardA syringe containing 1.47 mLmL of oxygen gas is cooled from 94.5 ∘C∘C to 0.8 ∘C∘C. What is the final volume Vf of oxygen gas?arrow_forwardI need help with part A and Barrow_forward
- Please answer the question Below.arrow_forwardWhat is the Qc for the following reaction, if a mixture contains gases with the following concentrations: CH4, 26 M; H2O, 24 M; CO, 26 M; H2 15 M, at a temperature of 760 0C? CH4(g) + H2O(g) CO(g) + 3H2(g)arrow_forwardThe pressure of a sample of neon gas at 171.2°C and 75.4 kPa was increased to 91.1 kPa. What is the new temperature of the neon gas in °C?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY