
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
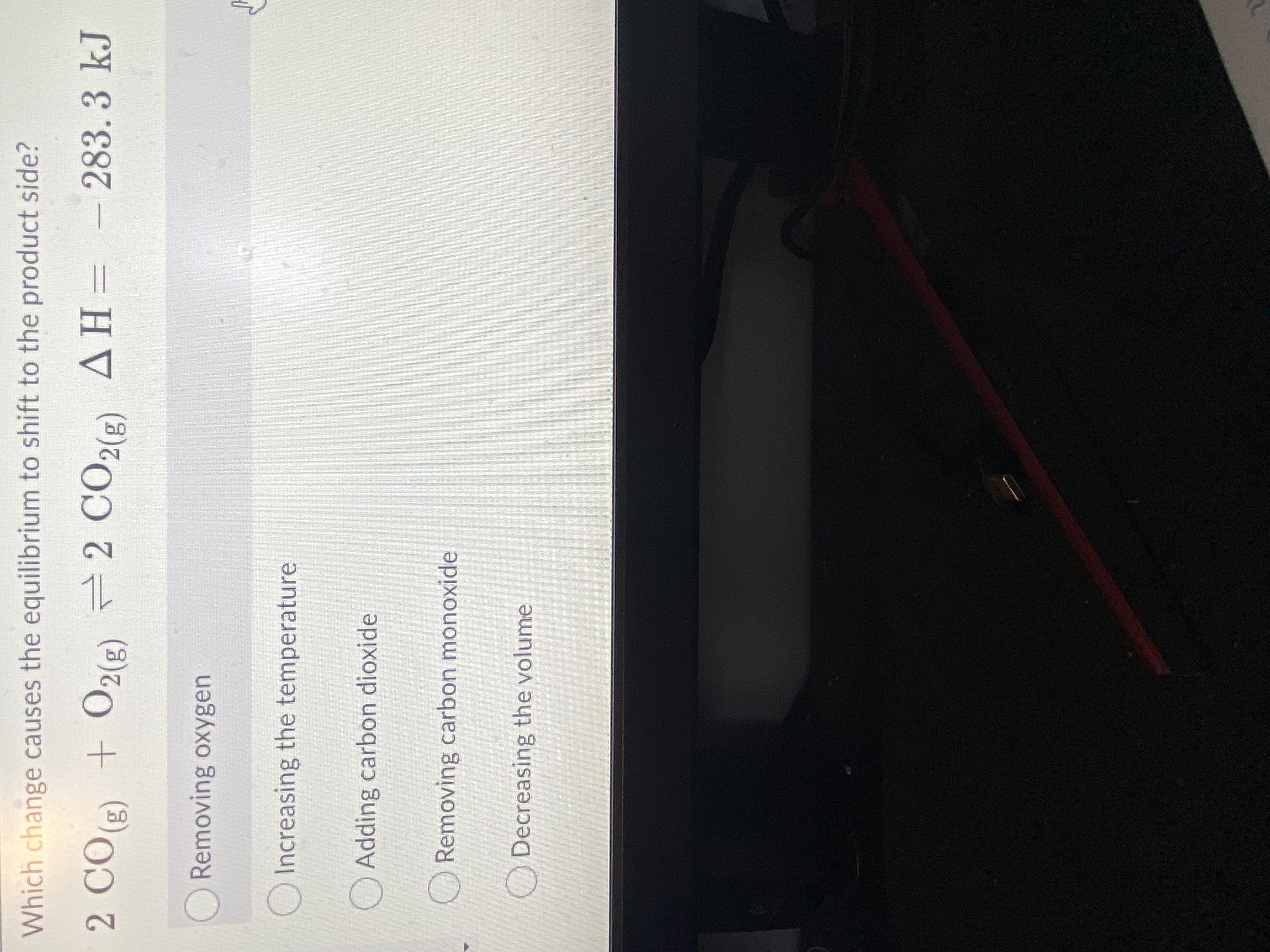
Transcribed Image Text:Which change causes the equilibrium to shift to the product side?
2 CO) + O2(2) 2 CO2(2) AH= -
(3)0)
283.3kJ
(3)2)
(3)2)
ORemoving oxygen
O Increasing the temperature
O Adding carbon dioxide
Removing carbon monoxide
Decreasing the volume
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 7 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Doarrow_forward2. Ozone can be created from oxygen gas (with an input of energy) via the following reaction: 30₂(8) = 203 (8) -2 If the equilibrium constant, K, is 1.02 x 10-24 for this reaction at a particular temperature and [0₂] = 3.10 x 10 M at equilibrium, what is [03] (in M to two decimal places) at equilibrium? A: 5.51 x 10 -15 (2) (028 DES (2)₂0+ (₂),025 = (8)¿025arrow_forward1-Please help show work and use significant figuresarrow_forward
- The reaction A2 + B2 =2 AB has an equilibrium constant Kc = 9.0. The following pictures represent reaction mixtures that contain A2 molecules (shaded) and B2 molecules (unshaded), and AB molecules. Which reaction mixture is at equilibrium? 0 618 ∞ 8 00 (1) (2) (4) reaction mixture (1) reaction mixture (2) reaction mixture (3) reaction mixture (4) O O O Oarrow_forwardAt a certain temperature, the equilibrium constant K for the following reaction is 734.: NO3(g) + NO(g) 2 NO₂(g) Use this information to complete the following table. ?arrow_forwardUse arrows (up / down) to indicate the affect of each of these disturbances (stresses) on the concentration of the reactants and products in this equilibrium: 9 KJ + 2 S02 9) + 02 (9) = 2 S03 (9A disturbance affect on affect on affect on ISO2] [02] ISO3] decrease [SO2] increase [02] increase [SO3] increase pressure decrease temp Period 4 CHEarrow_forward
- Alert for not submit AI generated answer. I need unique and correct answer. Don't try to copy from anywhere. Do not give answer in image and hand writingarrow_forward(#30) The Equilibrium Constant and the "ICE Chart" Phosphorus pentachloride gas is formed according to the reaction by adding chlorine gas to phosphorus trichloride gas. Then the reaction reaches equilibrium. The equilibrium constant, Kc, equals 49.0 at 230°C. If 0.500 mole each of the reactants are added to a 5-L container, what is the equilibrium composition at 230°C? (30a) In blank #1, type-in our answer for the concentration of phosphorus pentachloride in the product at equilibrium. The unit is Molarity, but enter only the value of your answer with two decimal places. (30b) In blank #2, type in your answer for the concentration of phosphorus trichloride at equilibrium with two decimal places. (30c) In blank #3, type in your answer for the concentration of chlorine gas at equilibrium with two decimal places.arrow_forwardDinitrogen tetroxide partially decomposes according to the following equilibrium: N204(g) = 2NO2(g) A 1.000-L flask is charged with 0.0300 mol of N204. At equilibrium, 0.0204 mol of N204 remains. Calculate the Kc for this reaction. (Enter only the numerical value to three significant figures without units.) Answer:arrow_forward
- For the following reaction, calculate all equilibrium concentrations if you start with 1.6 moles of nitrogen dioxide gas in a 4.0 L container. 2NO2(g) <====> N2O4(g) Keq = 1.5 at 250Carrow_forward(Q96) The equilibrium constant with respect to concentration (K) fer the reaction between nitrogen monoxide and oxygen gas to produce nitrogen diexide is 5.4 x 1013 at 25 C. What is the value ef the equilibrium constant with respect to partial pressures (Ke) under these same eenditiens? (2 sf) O 2.2 x 10^12 O5.4 x 10A13 1.3 x 19A15 @ 11x 1014 2.2x10 10arrow_forward302(9) = 203(g) O decreasing the volume will shift equilibrium in the direction of products O decreasing the volume will shift equilibrium in the direction of reactants no shift occurs Submit Request Answer Part B (6)o + (6)007 = (6)z007 decreasing the volume will shift equilibrium in the direction of products decreasing the volume will shift equilibrium in the direction of reactants no shift occurs Reguest Answer Submit Part C P.(g) + 50,(g) = P,010 (4) O decreasing the volume will shift equilibrium in the direction of products O decreasing the volume will shift alibrium in the direction of reactants no shift occurs Submit Reguest Answer Part D 2s0,(9) + 2H,0(g) = 2H,S(g) + 30,(g) O decreasing the volume will shift equilibrium in the direction of products decreasing the volume will shift equilibrium in the direction of reactants O no shift occurs O Oarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY