
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
What is the IR ANALYSIS FROM THIS MODEL
Please help
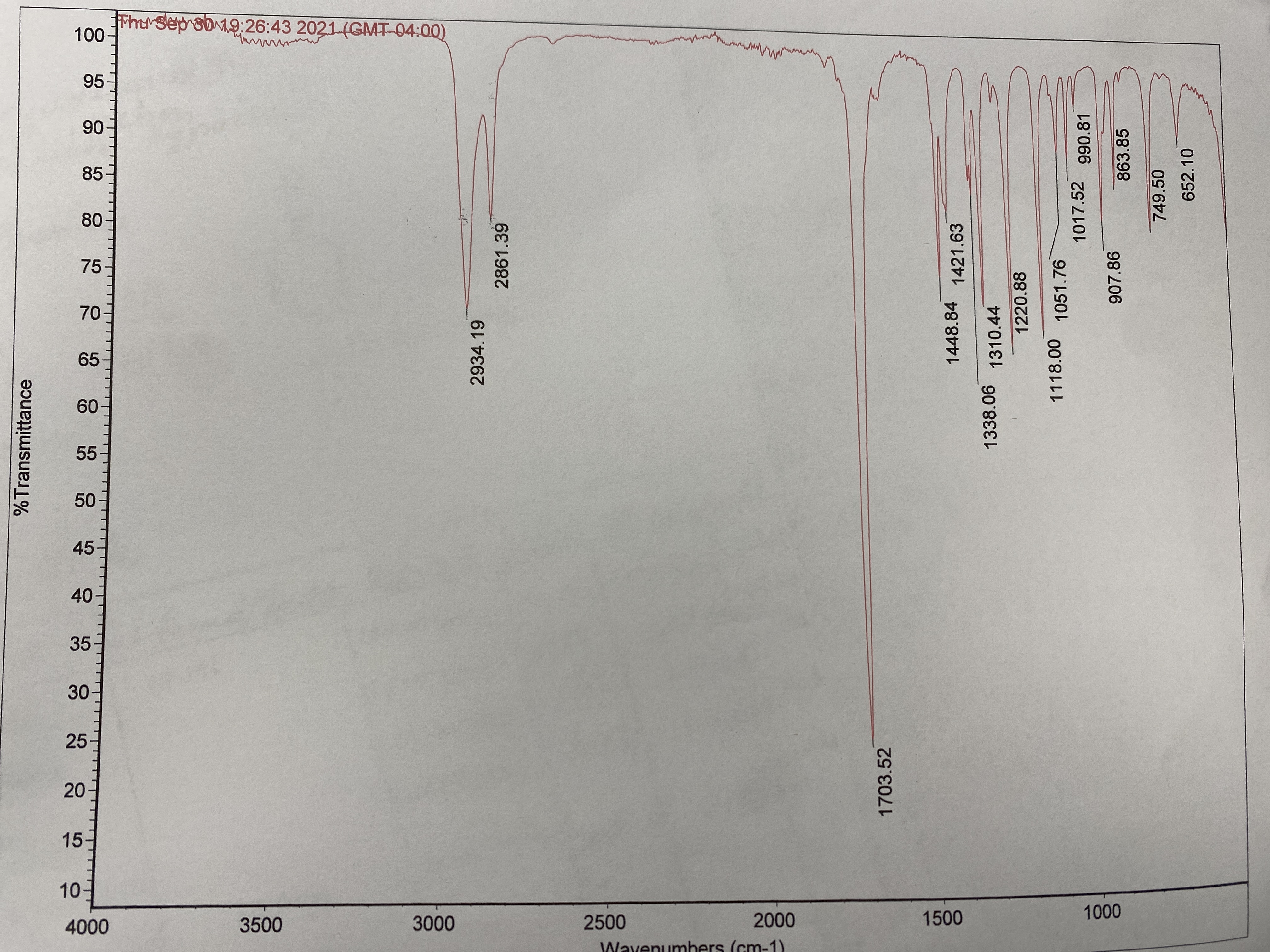
Transcribed Image Text:ThrSep sb19:26:43 2021-(GMT-04:00)
100
10
3500
3000
2500
2000
1500
1000
4000
Wayenumbers (cm-1)
%Transmittance
8 8 8 & 石 的 8 出品9 38 8 89
2934.19
2861.39
1703.52
1448.84
1421.63
1338.06
1310.44
1220.88
1118.00 1051.76
1017.52
990.81
907.86
863.85
749.50
652.10
Expert Solution
arrow_forward
Step 1
IR spectroscopy is a technique used in laboratories to identify the functional groups present in the compound.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- What is the Rf value and what is the advantage of calculating Rf value? Explain.arrow_forwardProject 2: Food Dye Spectroscopy What method will you use to assess the absorbance-concentration relationship? (The most obvious method is to see if there is a linear relationship between the absorbance and the concentration. What methods do you know that might reveal such a linear relationship?)arrow_forwardDumb question, just starting to learn about mass spectometry. I know we have to memorize the common radical fragments, M-15, M-29, etc, but lloking at the example, those numbers dont crrespond to the absorbance or the m/z, so for example, M-29. The absorbance for M-29 is at 100 , while the m/z is at 43. How is it designated to be M-29?arrow_forward
- . How can you calculate the concentration of a solution with UV-visible spectroscopy if you do not know the molar absorption? A. Add an internal standard. B. Use Beer's Law anyway. C. Add blue dye to the sample. D. Calculate the rate constant. E. Create a calibration curve.arrow_forwardPart A Mark with the green check to indicate that the given direction of electron flow in the following set of molecules using curved arrows notation is correct and with the red X label to indicate when incorrect. Drag the appropriate labels to their respective targets. ► View Available Hint(s) X X X H₂C X CH₂ H₂C Reset Help You labeled 2 of 7 targets incorrectly. Electrons can only be shown to migrate if there is an atomic p orbital to move them to. Moving a lone pair electrons to form a new π bond can only occur if the atom acceptingarrow_forwardCreate a calibration curve for the following 4 M concentrations. (0.16 M, 0.080 M, 0.040 M, and 0.016 M.) The data is in the attached image. Note* only use the absorbance points from the highlighted (highlighted in blue) column. It is the column with an absorbance of 442.6.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY