
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:B
gE
C.
N
H.
K.
%3D
P.
R.
5.
4.
7.
%2$
MacBook Pro
國2A
.06
.08
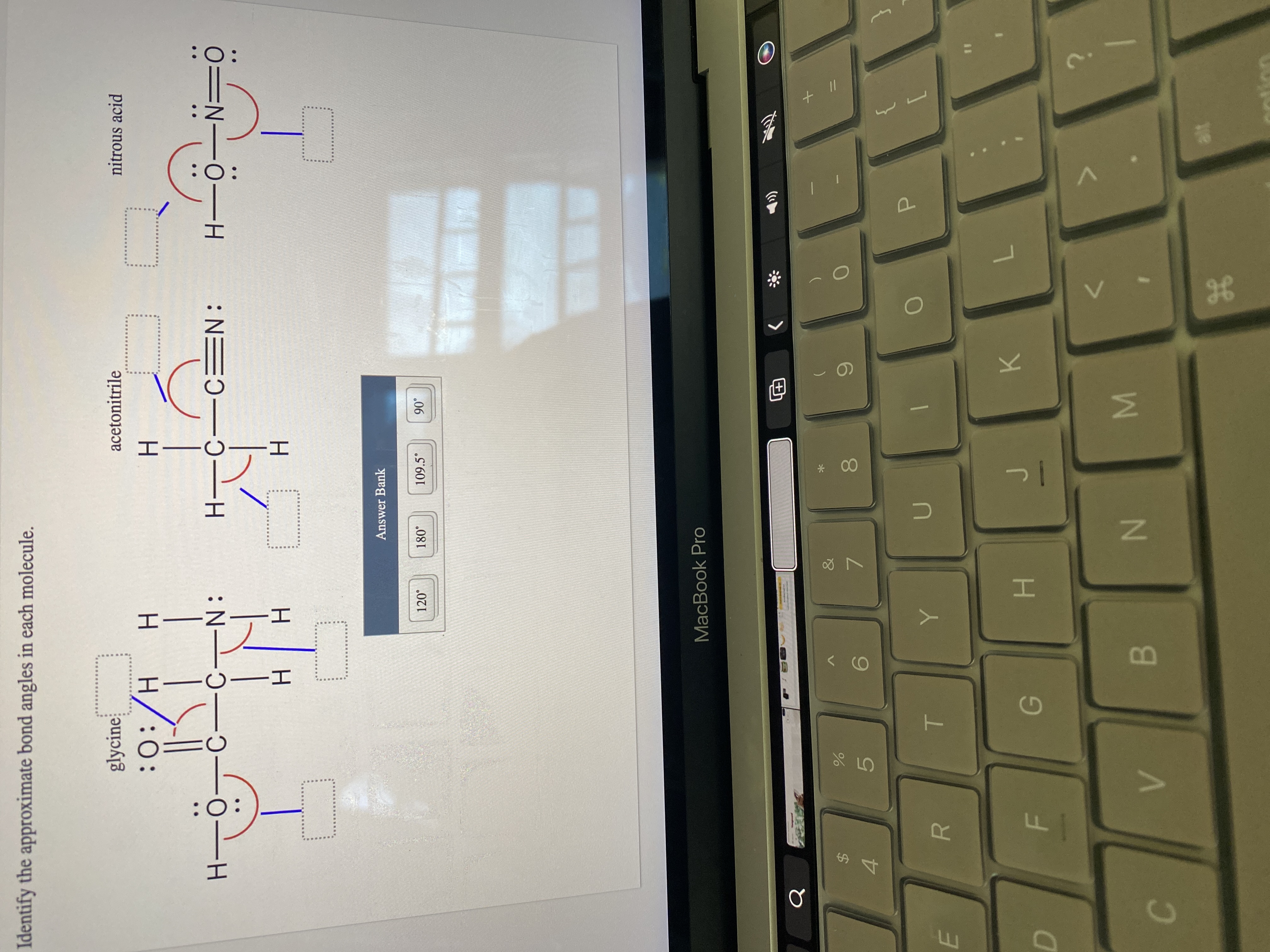
109.5°
Answer Bank
H.
H.
0=N-
0-H
:N=O-J-H
:N - -0-H
H.
H/:0:
nitrous acid
acetonitrile
glycine
Identify the approximate bond angles in each molecule.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Are these correct ?arrow_forwardDraw the Lewis Structure for each covalent system a-e below, and answer the question that follows: Electronegativites: H = 2.1 C = 2.5, B = 2.0N = 3.0, 0 = 3.5 S = 2.5, F = 4.0, Xe = 2.6 a. (OF 2 )Is this molecular POLAR or NONPOLAR? b. (XeF2) What is the geometry of this molecule? c. (SO 3 ^ 2- )What is the polyatomic ion geometry? (Assume "S" obeys octet) d.( CH 2 F 2 )Is this molecular POLAR or NONPOLAR? e.( BF 3 )Is this molecular POLAR NONPOLARarrow_forwardF3 Use the VSEPR model to predict the bond angles about each numbered atom. H H the 1 2 3.. H H Mastered The predicted angles about atom 1 are The predicted angles about atom 2 are The predicted angles about atom 3 are H Submit Answer $ 4 000 % 5 H F5 degrees. Retry Entire Group 9 more group attempts remaining F6 degrees. Cengage Learning Cengage Technical Support & 7 degrees. F7 * 8 if needed for this q DII F8 F9 F10arrow_forward
- н H. H- Н н -C-C-H H. H. For the given molecule, what is the hybridization at the atom numbered 3? Enter sp2,sp3,.... Submit Answer Tries 0/99 For atom 3, what is the bond angle? (degrees) Submit Answer Tries 0/99 :O:arrow_forwardIndicate the direction of polarity of each of the covalent bonds by placing the appropriate delta notation next to each end of the bond. 80 F3 $ 4 R F I CO V O-CI 0-F 998 CI-C S-H S-Cl % -N 5 T G B 6 MacBook Pro M Y H & 7 N U 8+ J Answer Bank * 8 DII FB M 8- ( 9 K DD F9 O V H I O L 4 F10 P ^. - ( F11 U { + [ = 21 F12 1 (1)arrow_forwardcould you please help me redraw this molecule and indicate all the bonds that have a dipole and the net dipole moment of the molecule, if one exists? Thank youarrow_forward
- 5-54 Using VSEPR theory, predict the molecular geometry of the following molecules. a. :0: :0::N:0:H b. O: H H:C:C:H Harrow_forward||| O ELECTRONIC STRUCTURE AND CHEMICAL BONDING Counting electron pairs in a Lewis structure with double or tripl... Below is the Lewis structure of the hydrazine (N₂H₂) molecule. 43°F Sunny H:N::N:H Count the number of bonding pairs and the number of lone pairs around the right hydrogen atom in this molecule. bonding pairs: lone pairs: Explanation Check X Q Search 3/5 K Jessica 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility ? olo Ar 83 4/26arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY