
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
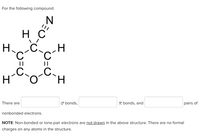
Transcribed Image Text:For the following compound:
H C
H.
.C
||
H.
There are
O bonds,
IT bonds, and
pairs of
nonbonded electrons.
NOTE: Non-bonded or lone-pair electrons are not drawn in the above structure. There are no formal
charges on any atoms in the structure.
Expert Solution
arrow_forward
Step 1
The sigma bond is represent by the single bond while the double bond or triple bond represents the pie bond.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- ents V Soul (TV series) O REPRESENTATIONS OF ORGANIC MOLECULES Drawing a skeletal structure from a Lewis structure Convert the Lewis structure below into a skeletal structure. Н—С— Н H. H- H- Н—С—Н H. HH H. H- Н—С—С—С—С—С—С—С- Н C=C H- H. H. H. Н—С— Н H. ct+ Click and drag to start drawing a structure. HIC CIH HICarrow_forwardDraw a second resonance form for the structure shown below. :0: -Ö: • Include all valencé lone pairs in your answer. • In cases where there is more than one answer, just draw one. Should you want to restart the exercise, the drop-down menu labeled == starting poin redraw the starting molecule on the sketcher. • In your structure, all second row elements should have a complete octet when possib 981 ANT - 0%. it's AIF 4arrow_forwardUse the molecular model kit to construct the following models, and then fill in the boxes for each molecule. When possible. show a resonance Lewis structure. Use formal charge, when possible to select the best structure. Follow example for CH4 below. Formula #VES CH4 8 NH3 H₂O CO₂ Lewis Structure H H-C-H H Resonance? (Y/N) Show N Electron Group & Bond Angle Tetrahedral 109° Molecular Polar? Geometry (Y/N) tetrahedral N VSPER Sketch H H 1 109.5° H H Hybridization sp³arrow_forward
- Please help me with these questions.arrow_forwardWhat is the correct Lewis structure for CH3CO2H? All lone pairs are drawn in (Hint: consider the “octet rule” :)arrow_forwarda. 01: Provide the formal charges for each atom in the molecule below, and answer the additional question (hint: none of the violate the octet rule). Format your answer as +2 or -3, for example. If there is no formal charge, then enter a zero (0). :0:4 b. 04: c. 05: H H d. Does this molecule have an overall charge (yes or no)? CH 3 21= CH3 Ö: hparrow_forward
- If both could be answered that would be great!arrow_forward1.44 Draw one valid Lewis structure for each compound. Assume the atoms are arranged as drawn. H HCC NO H a. CH₂N₂ HCNN H H b. CH₂NO₂ HCNO HO C. CH3CNO d. (CH₂CN) HC CN H 1.45 Draw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge. a. diethyl ether, (CH3CH₂)2O, the first general anesthetic used in medical procedures b. acrylonitrile, CH₂CHCN, starting material used to manufacture synthetic Orlon fibers c. dihydroxyacetone, (HOCH₂)₂CO, an ingredient in sunless tanning products d. acetic anhydride, (CH3CO)2O, a reagent used to synthesize aspirinarrow_forwardcan someone explain how Molecule B has a tertiary carbon ? Im having troubl with secondary and tertiary i tried to calculate the formal charges thinking that may have something to do with it but I ended up getting the wrong formal charge for molecule B, so now I'm confused.arrow_forward
- Question 16.b of 25 Classify and describe the properties of the following nitrogen containing compound. Provide a systematic name for this structure. N,N,N- 2- N- eth tri hex bi di CH3 prop N.N- CH3 pent but meth al amide an 1-arrow_forward3. The following shows all resonance structures for the following molecule. a. Draw in all implied lone pairs. b. Draw in curved arrows that show the flow of electrons, making sure the arrows show the precise starting point and destination of the electrons. Label each arrow as: lp →→→л (p=lone pair) c. d. Rank the resonance structures from most stable to least based on the number of formal charges and atoms that lack an octet of electrons. ol-of-o. B A D d-d-d-o E C F Garrow_forward3.A. Add all implied hydrogen atoms and any missing lone pairs of electrons. There are many possible ways to get a negative charge on carbon, but only one with filled octets.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY