
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:ion 1 of 26 >
O Macmillan Learning
Chemistry: Fundamentals and Principles
Davidson
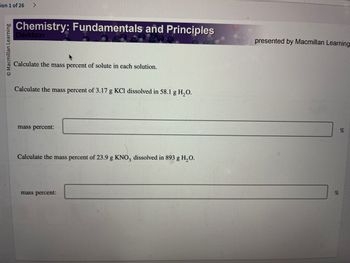
Calculate the mass percent of solute in each solution.
Calculate the mass percent of 3.17 g KCl dissolved in 58.1 g H₂O.
mass percent:
Calculate the mass percent of 23.9 g KNO3 dissolved in 893 g H₂O.
mass percent:
presented by Macmillan Learning
%
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What mass of a 4.00% NaOH solution by mass contains 15.0 g of NaOH?arrow_forwardWhat mass of HCl is contained in 45.0 mL of an aqueous HCl solution that has a density of 1.19 g cm-3 and contains 37.21% HCl by mass?arrow_forwardWhat mass of solid NaOH (97.0% NaOH by mass) is required to prepare 1.00 L of a 10.0% solution of NaOH by mass? The density of the 10.0% solution is 1.109 g/mL.arrow_forward
- Lead poisoning has been a hazard for centuries. Some scholars believe that the decline of the Roman Empire can be traced, in part, to high levels of lead in water from containers and pipes, and from wine that was stored in leadglazed containers. If we presume that the typical Roman water supply was saturated with lead carbonate, PbCO3 (Ksp = 7.4 1014), how much lead will a Roman ingest in a year if he or she drinks 1 L/day from the container?arrow_forwardSilver ions can be found in some of the city water piped into homes. The average concentration of silver ions in city water is 0.028 ppm. (a) How many milligrams of silver ions would you ingest daily if you drank eight glasses (eight oz/glass) of city water daily? (b) How many liters of city water are required to recover 1.00 g of silver chemically?arrow_forward34. For each of the following solutions, the number of moles of solute is given, followed by the total volume of the solution prepared. Calculate the molarity of each solution. a. 0.754 mol KNO; 225 mL b. 0.0105 in of CaCl; 10.2 mL c. 3.15 mol NaCl; 5.00 L d. 0.499 mol NaBr; 100. mLarrow_forward
- Lauryl alcohol is obtained from the coconut and is an ingredient in many hair shampoos. Its empirical formula is C12H26O. A solution of 5.00 g of lauryl alcohol in 100.0 g of benzene boils at 80.78C. Using Table 10.2, find the molecular formula of lauryl alcohol.arrow_forwardHow do we define the mass percent composition of a solution? Give an example of a solution, and explain the relative amounts of solute and solvent present in the solution in terms of the mass percent composition.arrow_forwardSolutions of hydrogen in palladium may be formed by exposing Pd metal to H2 gas. The concentration of hydrogen in the palladium depends on the pressure of H2 gas applied, but in a more complex fashion than can be described by Henry's law. Under certain conditions, 0.94 g of hydrogen gas is dissolved in 215 g of palladium metal (solution density = 10.8 g cm3). (a) Determine the molarity of this solution. (b) Determine the molality of this solution. (c) Determine the percent by mass of hydrogen atoms in this solution.arrow_forward
- Define a crystalline solid. Describe in detail some important types of crystalline solids and name a substance that is an example of each type of solid. Explain how the particles are held together in each type of solid (the interparticle forces that exist). How do the interparticle forces in a solid influence the bulk physical properties of the solid?arrow_forward6-21 Are mixtures of gases true solutions or heterogeneous mixtures? Explain.arrow_forward60. Suppose 325 in L of 0.150 M NaOH is needed for your experiment. How would you prepare this if all that is available is a 1.01 M NaOH solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
