
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Check all of the follow statements that are true of weak bases.
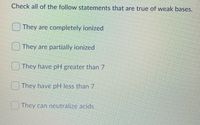
Transcribed Image Text:Check all of the follow statements that are true of weak bases.
They are completely ionized
They are partially ionized
They have pH greater than 7
They have pH less than 7
They can neutralize acids
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- the Ka for the weak acid HA is 5.82 *10^-7. calculate the Kb for conjugate base A- at 25 °C. report in scientific notation.arrow_forwardTable 12.3 (data) Substance pH Substance pH Substance pH Lemon Juice Household ammonia Baking soda Orange Juice Detergent (Laundry) Aspirin Milk Detergent (dishwashing) Buffered aspirin Salivia Table 12.4 (report) Substance pH Substances pHarrow_forwardWhen is it safe to assume that the change from initial concentration (+/- x) is small enough to be negligible/ignore when doing equilibrium calculations for weak acids and bases? Be specific!arrow_forward
- A 0.050 M monoprotic weak acid solution has a pH of 2.40. Calculate the pka of the acid. Express your answer with 2 places past the decimal point. « Previousarrow_forwardIdentify acid, base, conjugate acid, conjugate base, and the relative strengths of the species involved in the reactions.arrow_forwardArrange these acids from weakest to strongest based on their Ka value at 25 degrees C.arrow_forward
- Determine Kb for the nitrite ion, NO2- in a 0.10 - M solution this base is 0.0015% ionizedarrow_forwardPls help ASAP ON ALL ASKED QUESTIONS PLS PLSarrow_forwardIf the equilibrium pH of an aqueous solution is 1.72 and the solution is 0.380 M in formic acid, what is the percent ionization? (Two decimal places) Type your answer...arrow_forward
- Solve itarrow_forwardIf the pH of a solution is 3.3, then the pOH is ( Select ] and the solution is [ Select ]arrow_forwardIf the ?a of a monoprotic weak acid is 5.7×10^−6 ,what is the pH of a 0.27 M solution of this acid? Remember there are special significant figure rules you need to use when dealing with logs.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY