
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
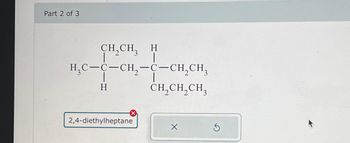
Transcribed Image Text:Part 2 of 3
CH,CH, H
|
H₂C-C-CH₂-C-CH₂CH₂
H
2,4-diethylheptane
CH₂CH₂CH₂
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the following of each problemarrow_forwardQ8 Conformational Analysis of Cycloalkanes In this question you will look at the conformations of the following molecule: CH3 C В F CH3 D -E A H3C F I II Energy Costs for Interactions in Alkane Conformers: Computed Energy Cost (kJ/mol)* 3.8 Interaction H+H eclipsed H+CH3 eclipsed CH3+CH3 eclipsed CH3+CH3 gauche H»CH(CH3)2 gauche H CH(CH:)2 eclipsed CH3+CH(CH3)2 gauche CH3+CH(CH3)2 eclipsed HeC(CH:)3 eclipsed CH3+C(CH3)3 gauche CH3+C(CH:)3 eclipsed H+I eclipsed I+CH3 gauche I+CH3 eclipsed "Values computed with Gaussian09 using B3LYP/6-31G(d) for structure optimization and B3LYP/6-311+G(2df,3p) for the conformation energy. The calculations were performed in the gas phase (no solvent). *This result for t-butyl is a bit peculiar given that the value is less than that for isopropyl, but there is an explanation that I can tell you about in office hours! 4.8 12.6 3.7 (0.0) 3.1 3.6 17.3 2.8 13.6 18.7 6.7 1.2 15.5 Steric Strain in Monosubstituted Cyclohexanes: 1,3-Diaxial strain Y (kJ/mol)…arrow_forwardFor each pair of compounds, please explain if they are identical. comstitutional isomers, enantiomers, or diastereomers.arrow_forward
- Identify the products (major and minor) of the following reaction:arrow_forwardDiethyl ether (CH3CH2OCH2CH3) and butan-1-ol (CH3CH2CH2CH2OH) are constitutional isomers with the same molecular formula, however their boiling points are very different. Which has the higher boiling point? O Diethyl Ether O Butan-1-olarrow_forward1-cyclopentylpropan-1-one benzaldehyde H NaOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY