
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Can someone tell me which reagent and substituent belongs in the equation? And why you chose that specific reagent ?
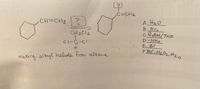
Transcribed Image Text:CHCHZ
CH=CH2?
CH2C12
A HzO
B Brz
C RBH/THF
D-NH2
FHo, HzOz, Hz o
making alkyl halide from alkene
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the alkene and reagent that are needed to synthesize the following compoundarrow_forward2) Give the reagents to make each product from the starting molecule. a) CH,=CHCH,CH,CH, b) c) Brarrow_forward---- Draw the structures of compounds X and Y in the box provided. Ph 1. Dihydroxylation 2. HIO4 HO, compound X compound Yarrow_forward
- Complete the mechanism for the keto-enol tautomerization shown using bonds, charges, nonbonding electron pairs, and curved arrows (forward reaction only). Do not delete any pre-drawn bonds, charges, or lone pairs. If you accidentally delete a vital part of the structure, click the undo button in the lower left. Step 1: keto form. Draw curved arrows. Step 2: complete the structure, then add curved arrows. Select Draw Rings More Erase Select Draw Rings More Erase H H - H :0: :0: :o: 1L :o:arrow_forwardWhich of these statements is correct? A) SN1 reactions but not Sy2 reactions create and break a bond to carbon B) Sy2 and E2 reactions create a carbon-carbon double bond C) E1 and E2 reactions must have a Lewis base D) The alkene in electrophilic additions of alkenes is the electrophile E) None of the above statements is correctarrow_forwardDraw the starting reactant that would produce this alkene when treated with this reagent. Ignore inorganic byproducts. Drawing Ph3P=CH CH3arrow_forward
- Draw the structure for the major product of each of the following reactions. If no reaction is possible then write NR. →> + L₂OH,H* 2CH CH OH, H → 2CH3CH2 Question 3 Draw the structure for the major product of each of the following reactions. If no reaction is possible then write NR. O CH Pd + H₂ CH3CH2CH2OH K2Cr2O7 CH3CH2CH2CH3 2 CH3CH2OH, Harrow_forwardThese are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". All of the carbon atoms of the products must come from the starting material(s)! ? suz يين (racemic)arrow_forwardThese are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". All of the carbon atoms of the products must come from the starting material(s)! hi ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY