
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
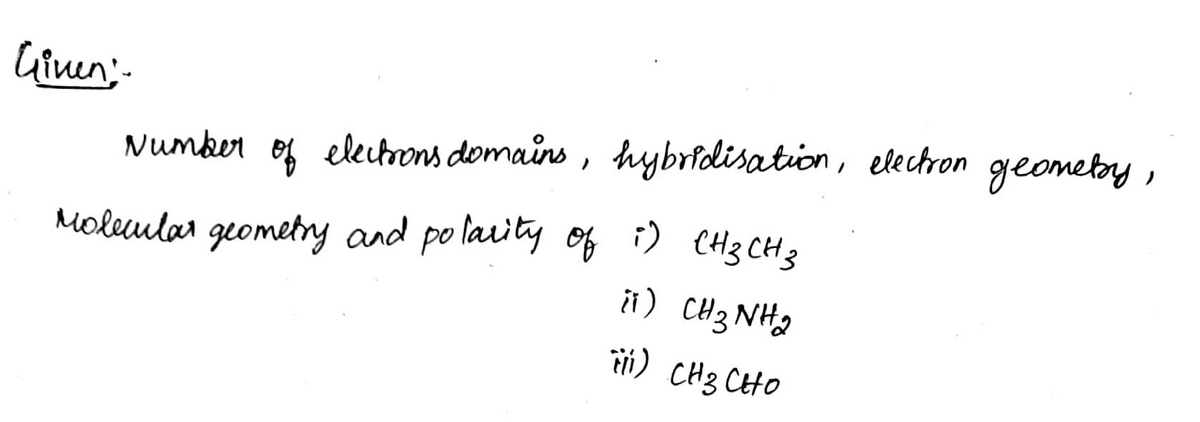
a) Number of electron domains. Explain your reasoning
b) Hybridization. Explain your reasoning
c) Electron geometry. Explain your reasoning
d) Molecular geometry. Explain your reasoning
e) Polarity - if they will be polar or not. Explain your reasoning.

Transcribed Image Text:### Chemical Structures Overview
The image displays three different chemical structures, each representing a distinct organic compound:
1. **CH₃CH₃**:
- **Ethane**: A simple alkane consisting of two carbon atoms single-bonded together, each bonded to three hydrogen atoms. It is a colorless and odorless gas under standard conditions and is commonly used as a fuel and a building block in the petrochemical industry.
2. **CH₃NH₂**:
- **Methylamine**: An organic compound derived from ammonia by replacement of one hydrogen atom with a methyl group. It is a primary amine and is utilized in the production of various pharmaceuticals and pesticides. It can also act as a building block in organic synthesis.
3. **CH₃CHO**:
- **Acetaldehyde**: An organic compound formed by the oxidation of ethyl alcohol. Acetaldehyde is an aldehyde with a functional group of –CHO and plays an important role in the chemical industry and biological processes. It is used as an intermediate in the synthesis of various chemicals.
These chemical structures are fundamental in organic chemistry and illustrate basic functional groups and molecular configurations in organic compounds. Understanding these structures aids in comprehending more complex chemical behavior and synthesis processes.
Expert Solution
arrow_forward
Given
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question: In the context of quantum mechanics and molecular orbital theory, how do non-bonding orbitals contribute to the stability of a molecule, and how do their electron density distributions affect the molecule's reactivity? Provide an example to illustrate your point.arrow_forward5.arrow_forwardb and c parts plsarrow_forward
- Use the References to access important values if needed for this question. Answer the following questions about the hybrid orbitals on the chlorine atom in the ClO3+ and ClO2+ molecular ions. What are the expected geometries of these ions? ClO3+: Central atom = Cl Type of hybrid orbitals on the chlorine atom = Number of hybrid orbitals used in overlap with atomic orbitals = Number of hybrid orbitals accomodating unshared electrons = Hybrid orbital geometry = Molecular geometry = ClO2+: Central atom = Cl Type of hybrid orbitals on the chlorine atom = Number of hybrid orbitals used in overlap with atomic orbitals = Number of hybrid orbitals accomodating unshared electrons = Hybrid orbital geometry = Molecular geometry =arrow_forwardWhat is the primary difference between valence bond theory (VB) and molecular orbital theory (MO)? A) VB determines the summation of mixed wave functions as hybrid orbitals; MO determines the sum of atomic orbital energy as energy level diagrams. B) VB determines the summation of mixed wave functions as energy level diagrams; MO determines the sum of atomic orbital energy as hybrid orbitals. C) MO determines the summation of mixed wave functions as energy level diagrams; VB determines the sum of atomic orbital energy as hybrid orbitals. D) MO determines the summation of mixed wave functions as hybrid orbitals; VB determines the sum of atomic orbital energy as energy level diagrams.arrow_forwardCan you explain how to draw hybridization diagrams? How do I determine the hybrid orbitals? How can I draw the energy diagram? Please explain using an example.arrow_forward
- Choose the molecules below that are polar. (Mark all that apply) CSe O3 CH4 NH3 H2S O2arrow_forwardAnswer the following question about acetonitrile (CH3C≡N:). Question: Determine the hybridization of both C atoms and the N atom ?arrow_forward3. Give the molecular formula for each molecule below. Give the hybridization and the electronic and molecular geometry for the highlighted atoms in the following structures. OH gl a) b) N C)arrow_forward
- How would we modify the figure if we were looking at PH3 rather than NH3?arrow_forwarda.) a= ~109° b=120° b.) a= ~120° b= ~109° c.) a= ~120° b=120° d.) a= ~109° b =~109°arrow_forwardAnswer the following question 1) Dry nitrogen gas (100.0 L) was bubbled through liquid chloroform, CHCI 3, at a given temperature and the evaporated chloroform condensed; its mass was then measured. Using the data below, calculate the heat of vaporization (kJ/mol) of chloroform? Temperature, °C Mass CHCl3 collected, g 12.19 72.05 27.03 131.7 2) Calculate the pOH after addition of 15.97 mL of 0.1762 M LIOH to 25.00 mL of 0.1582 M HBr. 3) Calculate the pOH after addition of 15.85 mL of 0.1209 M KOH to 25.00 mL of 0.1798 M CH 3CH 2CH ₂CO 2H. K a(CH 3CH 2CH 2CO₂H) = 1.500e-5 4)Calculate the pH after addition of 24.38 mL of 0.1148 M HCIO 4 to 25.00 mL of 0.1989 M CH 3CH 2NH 2. K b(CH 3CH 2NH2) = 6.400e-4. 5) What is the solubility of Sb 2S 3 if it's K sp is 1.700e-93?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY