
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
I'm not sure how to do this.
Change the bond between the two carbon atoms in each molecule to a double or triple bond as needed to complete the structure If the bond should remain a single bond, then you do not need to do anything to the bond. Do not change any other bonds in the molecules.
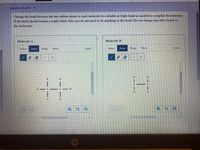
Transcribed Image Text:**Question 10 of 20**
**Instructions:**
Change the bond between the two carbon atoms in each molecule to a double or triple bond as needed to complete the structure. If the bond should remain a single bond, then you do not need to do anything to the bond. Do not change any other bonds in the molecules.
**Diagram Description:**
- **Molecule A:**
- Displays a structure with two carbon (C) atoms connected by a single bond.
- Each carbon atom is bonded to three hydrogen (H) atoms.
- **Molecule B:**
- Displays a structure with two carbon (C) atoms connected by a single bond.
- The left carbon atom is bonded to two hydrogen (H) atoms and the right carbon to three hydrogen (H) atoms.
**Tools Available:**
- Selection and drawing tools.
- Options to add carbon (C) or hydrogen (H) atoms.
- Erase options.
- Tools to zoom in or out for better visibility of the structure.
**Objective:**
Determine if the carbon-carbon bond in each molecule should be modified to a double or triple bond to satisfy the structural requirements. If the bond should remain unchanged, no action is needed.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a sketch of metallic bonds and label the metal cations and delocalized electrons. List one way metallic bonds and ionic bonds are similar. List one way metallic bonds and covalent bonds are similar. Use metallic bond theory to explain why metals are ductile (may be stretched into thin wire without breaking).arrow_forwardDraw the Lewis structure for CH2Se (C is the central atom) and answer the questions below. The steric number is ______, the electron pair geometry is _______ and the molecular geometry is ________ and has _______ double bonds.arrow_forwardDefine the difference between a polar and non-polar covalent bond. How do you determine if a molecule is polar or non-polar? Explain how a lone pair affects the bond angles of a substancearrow_forward
- Draw the Lewis structure for OSiS before answering the following questions (You do NOT submit a picture just answer the questions). Silicon is the central atom and all atoms obey the octet rule. WARNING: Do NOT USE the internet or other sources to find the structures. Use ONLY the rules taught in class. ALL atoms obey the octet rule Answer the following questions for the Lewis structure for OSiS , given that silicon is the central atom and all atoms obey the octet rule. Give answers as numbers (1,2,3 ...etc.) NOT words (one, two three etc.) How many double bonds exist in this structure How many electrons surround the silicon atom How many lone pairs are around the silicon atom How many lone pairs are around the sulfur atom How many lone pairs are around the oxygen atom How many electrons surround the Sulfur atomarrow_forwardFor each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds. element #1 element #2 calcium hydrogen sulfur carbon bromine bromine element pair will form a molecular compound molecular compound chemical formula 0 U 1 name 10 17 X 02 Śarrow_forwardHow bonds do H, O, N, and C form? Exam the diagrams of molecules and determine how many bonds H, O, N, and C each make. H. N-C-C NEN H-C=N H-N=C%3DO N-C-H H. N. II C-H N. N. H. RICIHO=U NIH HIarrow_forward
- REFER TO IMAGEarrow_forwardUse Lewis theory to determine the formula for the compound that forms between each of the following pairs of elements. Ca and Te Express your answer as a chemical formula. Mg and Br Express your answer as a chemical formula. Na and S Express your answer as a chemical formula. In and O Express your answer as a chemical formula.arrow_forwardFollowing is a molecule with polar bonds whose shape was obtained using the VSEPR theory. Specify the molecular shape of this molecule, and whether the molecule is polar or nonpolar. (Hint: In terms of polarity, see whether the dipoles in the molecule cancel or not. A molecule containing polar bonds can be nanpolar if the dipoles cancel each other. You can imagine the dipoles as ropes pulling on the central atom–If the pulls cancel each other, that is, the central atom cannot move, then the molecule is nonpolar. If on the other hand the opposite is true, then the molecule is polar.) O trigonal pyramidal shape, nonpolar O trigonal planar shape, nonpolar O tetrahedral shape, polar O trigonal pyramidal shape, polar O trigonal planar shape, polararrow_forward
- Electronegativity is the ability of an element to pull electrons towards itself in a bond. In a bond, the atom with the greater electronegativity will have a partial charge, and the atom with the lower electronegativity will have a partial charge. Fluorine has the higher/lower electronegativity and Francium has the higher/lower electronegativity. Metals like to gain/lose electrons, while nonmetals like to gain/lose electrons. Thus, metals have a electronegativity, while nonmetals have a electronegativity. a. negative b. positive c. lower d. higher e. lose f. gain g. the same h. none of thesearrow_forward1. Draw the best Lewis dot structure for the anion CCI in the correct molecular geometry [Include formal charges and lone pair electrons, and use dashed and solid wedge bonds if necessary 2. How many electron groups are present around the central atom and what is the electron group geometry? 3. What is the molecular geometry and ideal bond angles? 4. Is the molecule polar or nonpolar? If it is polar, draw a dipole moment arrow next to your structure to indicate the directionality of the dipole moment. Answers Edit View Insert Format Tools Table 12ptv Paragaph BIVA 2 Owordh AG3454jpg IMG 3450jpgarrow_forwardFor each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds. element #1 element #2 barium sulfur hydrogen carbon bromine chlorine element pair will form a molecular compound C 0 molecular compound chemical formula 7 name 0 0 X Śarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY