
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
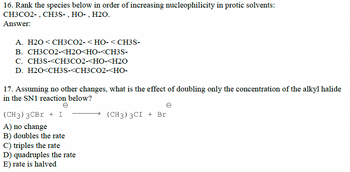
Transcribed Image Text:16. Rank the species below in order of increasing nucleophilicity in protic solvents:
CH3CO2-, CH3S-, HO-, H2O.
Answer:
A. H2O <CH3CO2-<HO-< CH3S-
B. CH3CO2-<H2O<HO-<CH3S-
C. CH3S-<CH3CO2-<HO-<H2O
D. H2O<CH3S-<CH3CO2-<HO-
17. Assuming no other changes, what is the effect of doubling only the concentration of the alkyl halide
in the SN1 reaction below?
(CH3) 3CBr + I
A) no change
B) doubles the rate
C) triples the rate
D) quadruples the rate
E) rate is halved
(CH3) 3CI + Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Find A and 8. CH3CH2COCI A Fecl3 C12 A Fecl3 O znCHg),HCI a CIarrow_forwardare parts c through f correct?arrow_forwardWhich represents the ring-flipped conformer of this compound? ||| Select one: O A. III OB. II O C. I O D. IV CH3CH₂ CH3 CH3CH₂ CH3CH₂ CH3CH₂ H3C- CH₂CH3 CH3 CH₂CH3 || CH₂CH3 G CH3 H3CH2₂C. IV CH3CH₂ CH3 G CH₂CH3arrow_forward
- Match the following cyclohexane to the correct lowest energy chair conformation. HO H3Carrow_forwardHCHO2 + H2O E--→CHO21 + H3O* when the [CHO,1] increases then which of the following will occur? a) [H2O] inc. b) [HCHO2] dec. c) [H3O*] inc. d) they all increase Tarrow_forwardOrganic chem homework help? Please?arrow_forward
- :&:&:&&;&;‘Msarrow_forwardDetermine the relationship between Structure A and Structure B in each row of the table. .O. Structure A :0: H H H H H :0-4 H-N-C=N: :0: H || HIC C C-H H H Structure B :Ö: C 0= H H / H H H :0-H H-C=N=N HHH H |||| HIC C-C-0: H O isomers O resonance structures O neither isomers resonance structures neither Relationship O isomers. O resonance structures O neitherarrow_forwardNAOH H2NNH2 5) 4) 3) Excess CH3OH H3O* РСС Br START HERE 1) Mg 2) CH3CHO 1) 2) 6)? Br KMNO4 DIBAL, 7) 8) H3C SOC22 Ag20 NH4OH Ph3P:CHCH2CH3 9) 14) 15) Benzene AlCl3 03 DMS C2H5OH, H3O* 10) 16) 17) HCN 11) H3O* 12) NH3 13)arrow_forward
- Chemistry Questionarrow_forwardH3C-CH,-CH,-CH-CH,- -c-CH3 е. OH IUPAC CH3 H,C-CH-CH,-C-CH2-CH3 IUPAC g. CH, H3C-CH-CH2-C-CH2-CH3 Derivedarrow_forward1. 2. = Ph 1. C 2. CO₂H I CH3 H3C-CH3 a = Proton transfer b Lewis acid/base C = Electrophilic addition Br₂ H₂O Ethanol CO₂H H Br Ph. H = OEt CH3 H₂C+0H1₂ H3 CH3 d E1 Elimination e = E2 Elimination f = SN1 Nucleophilic substitution g SN2 Nucleophilic substitution The rections above involve synthesis or reactions of alcohols and ethers. Identify the mechanism by which they proceed from among the mechanisms listed. Use the letters a- g for your answers. =arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY