
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Explain why tetrahydrofuran can solvate a positively charged species better than diethyl ether can.
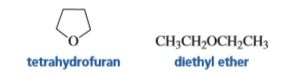
Transcribed Image Text:CH3CH,OCH,CH3
diethyl ether
tetrahydrofuran
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- name and draw the structural formulas for the 4 carboxylic acids with the molecular formula C5H10O2arrow_forwardCan 3-hexanol be easily made from hexanoic acid via reduction why or why not when trying to oxidize a primary alcohol to an aldehyde, why do you have to carefully choose your oxidizing agent and/or reaction conditions? Why is this not an issue in biological organism? Fully describe why amines are based in organic chemistryarrow_forwardWhat is the functional group of propanoic acid? How do we know if it's soluble in water or insluble in water using the flowchart that is attached. which test should be performed using the flowchart. Is propanoic acid a ketone, aldehyde, ester or alcohol and how did you know?what would be the most efficient series of solubility and functional group tests that would identify the functional group? Also what could be the sources of error in this experiment? What could occur that would give false positives, false negatives, or incorrect interpretations?arrow_forward
- Draw the skeletal ("line") structure of the smallest organic molecule that produces sodium hexanoate when reacted with NaOH. Click and drag to start drawing a structure. ✗ D: ☑ ค 5arrow_forwardTrue or false: The weaker a single bond in a molecule, the greater the chance it will be the site of a reaction (compared to stronger single bonds in the molecule).arrow_forwardWhich one of the following compounds has highest boiling point? HO || НО ОН IV НОarrow_forward
- Predict the color change if 1% ferric chloride (yellowish solution) is added to a test tube containing a mixture of ethanol and pure acetyl salicylic acid. purple to yellow yellow to dark blue light blue to pink yellow to purple no color change ————— Which of the following will be easily detected (not using any chemical test or instrument) if acetyl salicylic acid is heated in a flame? acetic anhydride acetic acid ethanol salicylic acid ————— Which of the following is NOT a volatile chemical? acetone hexanes benzoic acid methylene chloride petroleum ether ——— All of the following are corrosive chemicals EXCEPT hydrochloric acid sodium hydroxide benzoic acid sulfuric acid nitric acid potassium hydroxidearrow_forwardwhich is the correct option?arrow_forwardWrite the products of the reaction of diphenhydramine (a base) with the acid HCI shown below. H COCH₂CH₂NCH3 + HC1 CH3 Consider the chemical reaction from the previous question. Are the reactants or products more soluble in water? Briefly explain.arrow_forward
- How does the presence of an electronegative substituent such as Cl affect the acidity of a carboxylic acid?arrow_forwardWhich of the following compounds best dissolve in toluene? so, Na HO но, HO NH, a) b) d)arrow_forwardWhen butanoic acid (7.0 mL) is dissolved in methanol (20 mL) and heated with a catalytic amount of concentrated sulfuric acid, methyl butanoate (6.5 mL) is isolated. Calculate the per cent yield for the formation of this product. (Use a mole for the process)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY