
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
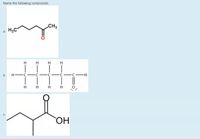
Transcribed Image Text:Name the following compounds.
CH3
H3C
a.
b.
H FC
C -C
-
H-
н н
С.
HO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Exta credit 1. Na0Me 2.H₂504 Δarrow_forward27 A chemist prepares a solution of sodium chloride (NaCl) by measuring out 0.50 g of NaCl into a 250. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Cl anions in the chemist's solution. do Be sure your answer is rounded to 2 significant digits. 18 Ar mol x10 L Submit Assignment Continue 2022 McGraw Hill LLC. AIl Rights Reserved. Terms of Use Privacy Center Accessibility Show All IMG-6095.jpg IMG-6096.jpg IMG-6097.jpg IMG-6098.jpg IMG-6099.jpg MacBook Air DD DII F12 F11 F10 F9 80 F7 F8 F6 F5 F4 esc F2 F3 F1 & % @ # 7 8 3 4 5 6 1 2 { P E R Y Q W %24arrow_forwardGasoline is composed of a variety of different liquid hydrocarbons, which do not separate as time passes. Gasoline is an example of a: A) heterogeneous mixture B) Chemical compound C) Chemical element D) Solutionarrow_forward
- You have a concentrated stock solution of 5 M NAOH and want to use it to produce a 300 mL solution of 3 M NaOH. What volume of water and stock solutions will you measure out to make this new solution? O a. 200 mL of water, 100 mL of NAOH stock O b. 100 mL of water, 200 mL of NaOH stock O c. 120 mL of water, 180 mL of NAOH stock d. 150 mL of water, 150 mL of NaOH stockarrow_forwardWhat area of study in chemistry is concerned with the heat transfers that occur during chemical reactions? b. thermochemistry c. calorimetry a. Stoichiometry The law of conservation of energy states that a. in any chemical or physical change, energy cannot be created or destroyed, only changed in form. b. heat changes occur during chemical and physical changes. c. energy is the capacity to do work or to supply heat d. there are two types of energy, kinetic and potential d. thermodynamicsarrow_forward1. When steam condenses, A. A physical change occurs. B. A chemical change occurs. C. Both physical and chemical changes occur. D. H20 molecules increase in number. E. H20 molecules decrease in number.arrow_forward
- The measure drug dosage is 0.10 mg drug for 1 kg body mass.arrow_forwardI Brightspace Walmart - Hiring Ce... < Q A N What is the final temperature (in °C) of 5.01 g of water (specific heat = 4.184 J/g °C) at 24.20 °C that absorbed 950.0 J of heat? + Bi 2 W S F3 3 E D C 144 $ 4 FS R F % 5 F6 T G 6 F7 B H F8 & 7 D Question 6 of 12 N F9 * 8 J F10 1 M ( 9 K F11 ) O L F12 P - DODE +/- PrtScr { 1arrow_forwardWhich of the following are chemical changes? a. Sawing a log b. Copper oxidizing c. burning a log d. grape juice fermenting e. glass breakinfarrow_forward
- Br Br 2. 1. Mg. Et₂0 3. H3O+ 1. Mg Et₂O 1. Mg Et₂0 2. 3. H3O+ Дон 2. H 3. H3O+ CH3 /Et₂0 0/E1₂0 Et₂0arrow_forward12. A 15.00g sample of a solid substance is placed in 100.0 g of water at 25°C, and all of the solid dissolves. Then another 2.00 g of the substance is added, and all of it dissolves. A final 2.00 g is added, and none of it dissolves. a. Is the first solution saturated, unsaturated, or supersaturated? b. Is the second solution saturated, unsaturated, or supersaturated? C. What can you tell about the final solution that is in contact with the solid?arrow_forwardWhat is the volume of 1.80 M NaCl solution that can be prepared by the dilution of a 50.0 mL of brine solution containing 32.0 wt% NaCl? (Assume the density of a brine solution is 1.10 g/mL and formula mass of NaCl=58.4 g/mol) a. 6.03 mL b. 167 mL C. 301 mL d. 438 mLarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY