
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Is this an Sn2 reaction? what is the mechanism?
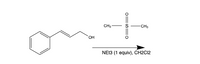
Transcribed Image Text:CH3
CH3
HO.
NE13 (1 equiv), CH2C12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the reaction. k₁ S P k₂ What effects are produced by an enzyme on the general reaction? AG for the reaction increases. The formation of the transition state is promoted. The reaction equilibrium is shifted toward the products. The concentration of the reactants is decreased. The activation energy for the reaction is lowered. The rate constant for the forward reaction (k₁1) increases.arrow_forwardDraw the products of the following reactions shown below in the picturesarrow_forwardGive the mechanism for the last step of the following reaction.arrow_forward
- (Q93) Which of the following best describes how a catalyst speeds up the rate of a reaction? O By decreasing the activation energy required to form the transition state O By decreasing the enthalpy difference between products and reactants O By removing the transition state Through acting as a supplemental reaction substrate O By ensuring that the reactant molecules do not collidearrow_forwardC. HINT: Hydrogenation Br + O ? O -arrow_forward(3) Evaluate the following uses of catalysis in terms of the goals of green chemistry (it is up to you if you want to refer to the 12 Principles or just the more general goals proposed by Dr. Lipke). Identify benefits as well as possible downsides. 2 a. A catalytic process that uses O, to oxidize an organic substrate dissolved in hexanes as a solvent, replacing a previous process that required the use of pyridine-N-oxide as an oxidant. b. A catalytic process that allows organic plant matter to be broken down into small organic molecules that can be used to produce fuels and fine chemicals that would otherwise be made from petroleum. c. A catalytic process that uses a mercury salt to catalyze the oxidation of methane to a methanol derivative, circumventing 2 a more traditional route using H, and CO to produce methanol as an intermediate.arrow_forward
- Identify the products of the 1,2-addition for the following reaction. OI O II O III O IV O III and IV Br Br + En Br Br + En || Br₂ CC14 ? Br Br + En ||| Br + En IVarrow_forwardIndicate the scheme and detailed reaction mechanism of the reactions:arrow_forwardWhat is the function of the enzyme lactase?arrow_forward
- Show the mechanism for the followingarrow_forwardCan you show the reaction mechanism step-by-step for C₃H₈O (l) → C3H6 (g) + H2O (l) propan-1-ol → propene + water Please show the electron movement and the way in which the bonds break. Can you please also show how many bonds there are and what type they are (eg. Sigma, Pi)? Thanks.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY