
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I put in the answer for compound A, (dimethyl‐lambda4‐sulfanylidene)cyclopropane,
and the product 9‐oxadispiro[2.0.44.13]nonane, in the boxes, but it says it's incorrect.
What am I not getting?
Thank you for your help!
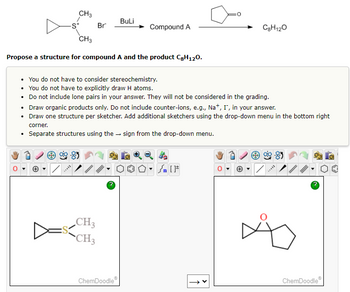
Transcribed Image Text:CH3
.
CH3
8
Br
Propose a structure for compound A and the product C8H120.
BuLi
CH3
CH3
Compound A
• You do not have to consider stereochemistry.
You do not have to explicitly draw H atoms.
•
Do not include lone pairs in your answer. They will not be considered in the grading.
• Draw organic products only. Do not include counter-ions, e.g., Na+, I, in your answer.
• Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right
corner.
• Separate structures using the sign from the drop-down menu.
ChemDoodleⓇ
Sn [F
0
R
C8H12O
+
/
f
ChemDoodleⓇ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Could someone help me name these compounds? Thanks!!!arrow_forwardAromatic compounds CH3C=CCH3 benzene CH3 CHCH3 1-ethyl-4-methylcyclohexane Other compounds Reset Helparrow_forwardWhich one of the following compounds has the highest melting point? O CH₂OC(CH₂)7CH-CH(CH₂),CH3 i O CHOC(CH₂)7CH-CH(CH2)7CH3 CH₂OC(CH₂)7CH=CH(CH₂)7CH3 triolein CH₂OC(CH₂)7CH=CH(CH2)5CH3 O CHOC(CH₂)7CH-CH(CH2)5CH3 CH₂OC(CH₂)7CH=CH(CH2)5CH3 tripalmitolein CH₂OC(CH₂)7CH=CHCH₂CH=CH(CH2)4CH3 сно иск CHOC(CH₂)7CH=CHCH₂CH=CH(CH2)4CH3 CH₂OC(CH₂)7CH=CHCH₂CH=CH(CH2)4CH3 trilinolein CH₂OC(CH2)12CH3 O CHOC(CH2) 12CH3 CH₂OC (CH2)12CH3 O trimyrsitinarrow_forward
- Give Detailed Solution (don't give Handwritten answer )arrow_forwardPlease make it clear and understandable thank you! -The last one is the letter: C)arrow_forwardIII Organic Functional Groups Predicting the reactants or products of amidation Predict the product of this organic reaction: OH + NH2 P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. 2/5 Tylerarrow_forward
- Chemistry: 1) SAM reacts with nucleophiles in an SN2-like reaction in our body for a variety of important biosynthetic reactions. Below on the molecule of SAM, please circle and label which part of the molecule would act as the electrophile, and which part of the molecule is the leaving group. (5 pts) HO NH2 NH2 N N CH 3 N Z -S: + 'OH OH S-Adenosyl methioninearrow_forwardA compound was found to have the molecular formula C5H12O and could be any of the compounds shown below. To determine the identity of the unknown, a series of chemical tests were conducted. Assign each test result to the correct compound. Further, the oxidation product of the unknown was able to turn blue litmus paper into red. Determine its identity. Type in the blanks the set of CAPITAL LETTERS corresponding to your answer. OH Į Test MSH reagent/result CrO3, H₂SO4 Conc. HCI, ZnCl₂ Aq. FeCl3 OH Reaction with Na metal (evolution of H₂) Cpd (1): SHM Cpd (2): + ++ ++ IDENTITY OF THE UNKNOWN: OH Xx MHS Cpd (3): + HMS Cpd (4): +++ +++arrow_forwardUsing the periodic table of elements answer the following: A) either 2-methyl propane is converted to 2-bromo-2methyl propane by reaction Br2 in presence of UV light B) or 2-methyl propensity is converted to 1-chloro-2methyl-2-propanol by reaction with an aqueous solution of chlorine (Cl2)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY