
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Study and classify the following reactions as S (substitution), E (elimination) or A (addition). Explain your answer.
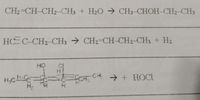
Transcribed Image Text:CH2=CH-CH2 CH3 + H2O → CH3-CHOH-CH2-CH3
HC C-CH2 CH3 → CH2=CH CH2-CH3 + H2
HỌ
CH + HOCI
H.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 100 Addition Substitution Esterification Elimination 1. 2. C₂H4(g) 4. + H₂(g) → C₂H6 (g) C₂H5OH (1) → C₂H4(g) + H₂O(1) 3. C6H6 (1) + Br2 (1)→ C6H₂Br (1) + HBr (g) CH3COOH()+CH₂OH(1)→ CH3COOCH3 (1) + H₂O (1)arrow_forwardThis is organic chemistry. I’m kind of lost which one is wrong.arrow_forwardChapter 11 - Attempt 1 Problem 11.22 - Enhanced - with Feedback 5 of 8 > Review I Constants I Periodic Table You may want to reference (Pages 375-376) Section 11.4 while completing this problem. Part B Enter the balanced chemical equation for the complete combustion of each of the following compounds. cyclohexane (use the molecular formula for the hydrocarbon) Express your answer as a chemical equation, using the molecular formula for the hydrocarbon. Omlt the phases in your answer. ΑΣφ ? DA chemical reaction does not occur for this question. Submit Previous Answers Request Answer Next * Previous MacBook Air F10arrow_forward
- 6. One of the most common chemical reaction mechanisms used in living systems is the nucleophilic substitution reaction. What is nucleophilic substitution? What are the two different types of nucleophilic substitutions? What is the main difference in the products resulting from these reactions? Describe them with help of generic structural sketches.arrow_forward7. Consider the following three step reaction. [4 (a) Add curved arrows in Step [1] to show the movement of electrons. H Lö: HÖ [1] + H-Ci: (b) Use the curved arrows drawn in Step [2] to identify the structure of X. X is converted in Step [3] to phenol and HCI. [2] + H-CI: [3] phenolarrow_forward3. Draw mechanism and energy profile diagram when chlorine reacts with propane. Write a mechanism using half arrows for initiation, propagation and termination steps. Draw an energy profile diagram similar to question #2, two curves for major and minor products. Indicate minor and major products. Which product is more stable and why? Explain is Hammond's postulate?arrow_forward
- Question 4 (b). Chlorobenzene, also known as monochlorobenzene, benzene chloride, chlorobenzol, and phenyl chloride, is used in the production of chloronitrobenzene and diphenyl ether, in rubber intermediates; as a solvent in adhesives, paints, waxes, and polishes; and as an inert solvent. Ms Bontle Makgatho, a Bsc I student at Sefako Makgatho Health Sciences University is doing an internship at SKY PAINT, a company based in Gezina, Pretoria North. One of her duties involves the synthesis of Chlorobenzene using benzene as a starting material. (i). Draw a step wise mechanism for the synthesis of chlorobenzene using Benzene as the starting material (ii). Benzene is a well-known aromatic compound, what are the characteristic of an aromatic compound (list four) Save and Submit to save and submit. Click Save All Answers to save all answers.arrow_forwardThese are organic chemistry questions can you solve these 3 questions with its mechanismarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY