
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
-
- Calculate the degree of unsaturation.
- Assign the relevant peaks in the IR spectrum.
- Assign the peaks in the 13C NMR spectrum.
- Assign the peaks in the 1H NMR spectrum.
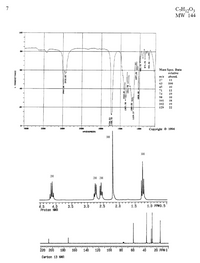
Transcribed Image Text:7
C,H1,03
MW 144
%3D
Mass Spee. Data
relative
abund.
27
11
43
100
45
71
74
10
19
10
98
101
18
102
19
129
22
Ja
Copyright O 1994
VENUMBERS
3H
3H
2H 2H
2.5
2.0
1.0 PPMO.5
4.5
4.0
Proton NMA
3.5
3.0
1.5
20 200
180
160
140
120
100
80
40
20 PPM O
Carbon 13 NHA
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How can the major product be identified in the infrared spectrum? A medium strong peak at 1674 cm1 O A small peak at 1648 cm1 O A strong peak at 1038-1050 cm1 A broad peak from 3200-3500 cm1 O A strong peak at 1649 cm1arrow_forwardWhat is the molecule that would produce the following H-NMR spectrum? 요 Singlet 2H Хинг O a. 3 only O b. 1 only O c. 4 only O d. 5 only 2 Singlet 9H Broad singlet 2H 2 PPM OH NH2arrow_forwardCalculate the degree of unsaturation. Assign the relevant peaks in the IR spectrum. Assign the peaks in the 13C NMR spectrum. Assign the peaks in the 1H NMR spectrum.arrow_forward
- is commonly used as an internal reference in NMR spectroscopy; its signal is assigned 5 = 0 in 13C NMR spectroscopy.arrow_forwardCalculate the degree of unsaturation. Assign the relevant peaks in the IR spectrum. Assign the peaks in the 13C NMR spectrum. Assign the peaks in the 1H NMR spectrum.arrow_forwardCalculate the degree of unsaturation. Assign the relevant peaks in the IR spectrum. Assign the peaks in the 13C NMR spectrum. Assign the peaks in the 1H NMR spectrum.arrow_forward
- How many peaks in NMR will be given by the Hydrogen b of the upper image and how many by the Hydrogen b of the lower image and how do I find it?arrow_forwardHow could 1H NMR spectroscopy be used to distinguish betweencompounds X and Y?arrow_forwardHow many of the molecules below would have 3 signals in the ¹H NMR spectrum? Do not consider split signals as seperate signals. CH3 0000 0123 0 H₂ CH3 нство да CH3 H CH3 Тarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY