
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
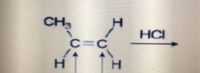
Transcribed Image Text:CH
HCI
C=C
H.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You set up a titration in the lab to determine the concentration of an HCl solution. What is the molarity of an HCl solution if 0.0100 L of 0.245 M NaOH soln’ are required to neutralize 0.0150 L HCl? 2.25 M 0.163 M 1.34 M 0.245 Marrow_forwardb. NaOH (aq) →→→ Na* (aq)+OH- (aq)arrow_forwardWhat is the product of the reaction series shown below? NH2 CI NANO,, HCI CUCN LIAH,, H,O H,0,0°C CN NH NH2 .CI .CI .CI A. В. C. D. O B O D MacBook Pro G Search or type URL & * 4 5 7 8 Y U o o O %23arrow_forward
- A 1.0 M solution of HNO3 was used to neutralize 100.0 ml of 0.20 M Ba(OH)2. b. How many ml of 1.0 M HNO3 are needed to neutralize 100.0 ml of 0.20 M Ba(OH)2?arrow_forward. HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)What are the spectator ions when the reaction above is written in total ionic form. a. H+, OH- b. Na+, Cl- c. H+, OH-, H2O d. H+, Cl-, Na+, OH-arrow_forwardD * Question Completion Status: Moving to the next question prevents changes to this answer. Question 1 D 5 points What is the percent of CaCO3 in an antacid given that a tablet that weighed 1.3198 g reacted with 50.00 mL of 0.4486 N HCI that subsequently required 3.72 mL of 0.1277 N NaOH for back titration? NB. Write the only value without units, eg: If the correct answer is 5.5%, write 5.5. A Moving to the next question prevents changes to this answer. MECER 6 Question 1 of 27 ENG US Save Answer Question 1 of 27 > 14:29 2022/10/05arrow_forward
- What’s the answer in Marrow_forwardWhat mass (in kg) of H(OH) can be produced if 56mL of 0.982M H2C2O4•H2O solution reacts completely with excess NaOH? __ H2C2O4•H2O + __NaOH = __ Na2C2O4•H2O + __ H(OH)arrow_forwardA 45.0 mL solution of LiOH is neutralized with 36.5 mL of 0.350 M HCl. What is the concentration of the original LiOH solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY