
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Q2
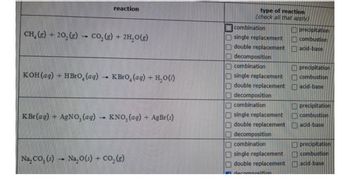
Transcribed Image Text:CH, (g) - 20, (g)
H
CO₂(g) + 2H₂O(g)
KOH(aq) + HBrO, (ag)
reaction
IND
KBrO, (aq) + H₂O()
K Br(ag) — AgNO,(aq) • KNO, (aq) + AgBr(s)
H
Na₂CO₂ (s) → Na₂O (3) + CO₂(g)
type of reaction
(check all that apply)
combination
single replacement
double replacement
decomposition
combination
single replacement
double replacement
decomposition
combination
single replacement
double replacement
decomposition
combination
single replacement
double replacement
darn
precipitation
combustion
acid-base
precipitation
combustion
acid-base
precipitation
combustion
acid-base
precipitation
combustion
acid-base
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An electric current of 25.80 A transports 0.30 kC of charge. Calculate the time this took. Be sure your answer has the correct unit symbol and the correct number of significant digits. x10arrow_forwardWhich of the following has the smallest value of IE1? O S Se Tearrow_forward1 1 J 1 MJ 1 m J Edlanation = 10 = 10 = 10 = 10 Check -1 3 J J J J by filling in the missing prefix μ Xarrow_forward
- a = -bcd In order to solve the equation above for d, you must multiply both sides of the equation by the same expression: ax = -bcd x x The resulting equation is: d =arrow_forwardH3C = H₂ Pd/C H3C- H ind Mes H 4arrow_forward(0.0050 x 28000.0) + (2819 × 15) Express your answer to the appropriate number of significant digits. να ΑΣφ ? (0.0050 x 28000.0) + (2819 × 15) = Submit Previous Answers Request Answer X Incorrect; Try Again; One attempt remaining Part D 861 x [1255 – (3.65 x 101)] Express your answer to the appropriate number of significant digits. ? 861 x [1255 – (3.65 x 101)] = Submit Previous Answers Request Answer X Incorrect; Try Again; 2 attempts remainingarrow_forward
- Calculate the electrostatic force of repulsion for two protons separated by 93.0 pm. The charge of a proton is q = 1.6 x 10-1⁹ C. Express your answer to two significant figures and include the appropriate units. Fel = μA 2.7.10 6 Submit N Previous Answers Request Answer ? X Incorrect; Try Again; 5 attempts remaining Use the equation Fel = K(Q₁ Q₂/d²) to find the electrostatic force between the two particles. Q₁ and Q2 are the charges on the particles (charge of a proton here), and d is the distance of separation. The constant is given in the introduction and has the units Nm²/C². This means that the distance must be converted from picometers to meters using the relation 1 pm = 1 × 10¯ m. -12arrow_forwardWhat is the boiling point of the unknown substance Xin K(Kelvin)? X(t) = X(g) AH-(kJ mol1) S°J mol1K1) X(g) -82.0 238 | X({) | -101 144 Express your answer in decimal notation rounded to three significant figures.arrow_forwardWhen an object with an electric charge of −3.0μC is 8.0cm from an object with an electric charge of 6.0μC, the force between them has a strength of 25.28N. Calculate the strength of the force between the two objects if they are 24.cm apart. Round your answer to 2 significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY